How To Identify Strong And Weak Acids And Bases
Muz Play
Mar 19, 2025 · 6 min read
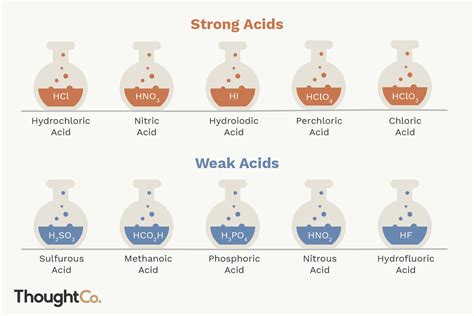
Table of Contents
How to Identify Strong and Weak Acids and Bases
Understanding the strength of acids and bases is fundamental in chemistry. Whether you're a student tackling general chemistry or a seasoned researcher working with complex reactions, knowing how to distinguish strong from weak acids and bases is crucial. This comprehensive guide will delve into the concepts, provide clear identification methods, and explore real-world applications.
Understanding Acids and Bases
Before we dive into identifying strong and weak acids and bases, let's establish a solid foundation. Acids and bases are defined by their behavior in aqueous solutions (dissolved in water).
Arrhenius Definition
The simplest definition, the Arrhenius definition, states:
- Acids: Substances that increase the concentration of hydrogen ions (H⁺) in solution. Think of them as H⁺ donors.
- Bases: Substances that increase the concentration of hydroxide ions (OH⁻) in solution. They are OH⁻ donors.
While useful, the Arrhenius definition has limitations. It doesn't encompass all substances exhibiting acidic or basic properties.
Brønsted-Lowry Definition
A more comprehensive definition is the Brønsted-Lowry definition:
- Acids: Substances that donate protons (H⁺).
- Bases: Substances that accept protons (H⁺).
This definition expands the scope to include substances that don't contain hydroxide ions but still act as bases by accepting protons. For instance, ammonia (NH₃) is a Brønsted-Lowry base.
The Crucial Difference: Strength vs. Concentration
It's vital to distinguish between the strength of an acid or base and its concentration.
- Strength: Refers to the degree of ionization or dissociation in water. Strong acids and bases completely ionize or dissociate, while weak ones only partially do so.
- Concentration: Refers to the amount of acid or base present in a given volume of solution. A solution can be highly concentrated but still contain a weak acid or base.
Confusing these two concepts leads to significant errors in chemical calculations and predictions.
Identifying Strong Acids
Strong acids completely dissociate into their constituent ions in water. This means that virtually all of the acid molecules break apart into hydrogen ions (H⁺) and their conjugate base anions. Memorizing a short list is the most effective method:
The Six Common Strong Acids:
- Hydrochloric acid (HCl): Found in stomach acid and used in various industrial processes.
- Hydrobromic acid (HBr): Used in the synthesis of organic compounds.
- Hydroiodic acid (HI): Also used in organic synthesis and some analytical procedures.
- Nitric acid (HNO₃): A strong oxidizing agent used in fertilizer production and metal etching.
- Sulfuric acid (H₂SO₄): A highly corrosive acid with widespread industrial uses, including in the production of fertilizers and batteries. Note that it dissociates in two steps, but the first step is essentially complete, making it a strong acid.
- Perchloric acid (HClO₄): A very strong oxidizing agent used in various chemical processes.
If an acid is not one of these six, it's considered a weak acid.
Identifying Strong Acids in Reactions
In chemical equations, the complete dissociation of a strong acid is represented by a single arrow (→), indicating a virtually irreversible reaction. For example:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
This shows that hydrochloric acid (HCl) completely dissociates into hydrogen ions (H⁺) and chloride ions (Cl⁻) in aqueous solution.
Identifying Weak Acids
Weak acids only partially dissociate in water. This means that a significant portion of the acid molecules remain undissociated in solution, existing in equilibrium with their ions. The extent of dissociation is represented by an equilibrium constant, Ka (the acid dissociation constant). A smaller Ka value indicates a weaker acid.
Examples of Weak Acids:
- Acetic acid (CH₃COOH): Found in vinegar.
- Citric acid: Found in citrus fruits.
- Carbonic acid (H₂CO₃): Formed when carbon dioxide dissolves in water.
- Hydrofluoric acid (HF): Used in etching glass.
- Phosphoric acid (H₃PO₄): Used in fertilizers and food additives.
Identifying Weak Acids in Reactions
The partial dissociation of a weak acid is represented by a double arrow (⇌), indicating a reversible reaction. For example:
CH₃COOH(aq) ⇌ H⁺(aq) + CH₃COO⁻(aq)
This shows that acetic acid (CH₃COOH) only partially dissociates into hydrogen ions (H⁺) and acetate ions (CH₃COO⁻). A significant amount of undissociated acetic acid remains in solution.
Identifying Strong Bases
Strong bases completely dissociate in water, producing a high concentration of hydroxide ions (OH⁻). Like strong acids, there's a relatively small group to memorize:
Common Strong Bases:
- Group 1 hydroxides (alkali metal hydroxides): These include lithium hydroxide (LiOH), sodium hydroxide (NaOH), potassium hydroxide (KOH), rubidium hydroxide (RbOH), and cesium hydroxide (CsOH). These are highly soluble in water and completely dissociate.
- Some Group 2 hydroxides (alkaline earth metal hydroxides): While not all Group 2 hydroxides are strong bases, barium hydroxide (Ba(OH)₂) and strontium hydroxide (Sr(OH)₂) are considered strong bases due to their relatively high solubility and complete dissociation in water. Calcium hydroxide [Ca(OH)₂] is only partially soluble, limiting its strength.
Identifying Strong Bases in Reactions
Similar to strong acids, the complete dissociation of a strong base is represented using a single arrow:
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
This indicates the complete dissociation of sodium hydroxide (NaOH) into sodium ions (Na⁺) and hydroxide ions (OH⁻).
Identifying Weak Bases
Weak bases only partially dissociate in water, resulting in a lower concentration of hydroxide ions (OH⁻) compared to strong bases. Their extent of dissociation is characterized by Kb (the base dissociation constant). A smaller Kb value indicates a weaker base.
Examples of Weak Bases:
- Ammonia (NH₃): Used in various cleaning products.
- Many amines (organic compounds containing nitrogen): These compounds often act as weak bases due to the presence of a lone pair of electrons on the nitrogen atom, allowing them to accept a proton.
- Pyridine: An organic heterocyclic compound often used as a solvent and reagent.
Identifying Weak Bases in Reactions
Weak bases, similar to weak acids, are represented by a double arrow in chemical equations:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
This illustrates the partial reaction of ammonia (NH₃) with water, producing ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). The equilibrium lies far to the left, meaning most of the ammonia remains undissociated.
Practical Applications and Considerations
The ability to identify strong and weak acids and bases is essential in numerous applications:
- Titrations: Knowing the strength of the acid or base being titrated is crucial for accurate calculations. Strong acid-strong base titrations have sharp equivalence points, while weak acid-weak base titrations have less distinct ones.
- Buffer Solutions: Buffer solutions, which resist changes in pH, are often composed of a weak acid and its conjugate base or a weak base and its conjugate acid. Understanding the strength of these components is crucial for designing effective buffers.
- Industrial Processes: Many industrial processes, such as the production of fertilizers, pharmaceuticals, and cleaning agents, rely on carefully controlled acid-base reactions. Proper identification of the strengths of these substances is vital for optimizing the processes and ensuring safety.
- Environmental Monitoring: The pH of water bodies and soil is a critical factor in environmental health. Identifying the presence and strength of acids and bases helps in understanding and managing environmental pollution.
- Biological Systems: Many biological processes rely on carefully controlled pH levels. The strengths of acids and bases play a critical role in maintaining these pH levels.
Conclusion
Identifying strong and weak acids and bases is a cornerstone of chemistry. By understanding the definitions, learning to recognize common strong acids and bases, and appreciating the concept of equilibrium in weak acid and base dissociations, you gain a powerful tool for analyzing chemical systems and predicting their behavior. Remember, mastering this skill is key to success in chemistry, whether in academic studies or practical applications. Further exploration into acid-base equilibrium constants (Ka and Kb) and pH calculations will solidify your understanding.
Latest Posts
Latest Posts
-
Do Protons Have About The Same Mass As Neutrons
Mar 19, 2025
-
What Is Monomer Of Nucleic Acids
Mar 19, 2025
-
Inverted Vs Everted Palindromic Dna Sequence Example
Mar 19, 2025
-
What Makes Up Most Of The Mass Of An Atom
Mar 19, 2025
-
Policy Implementation Refers To The Bureaucratic Function Of
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Identify Strong And Weak Acids And Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
