Antimicrobial Sensitivity Testing Kirby Bauer Method
Muz Play
Mar 19, 2025 · 7 min read
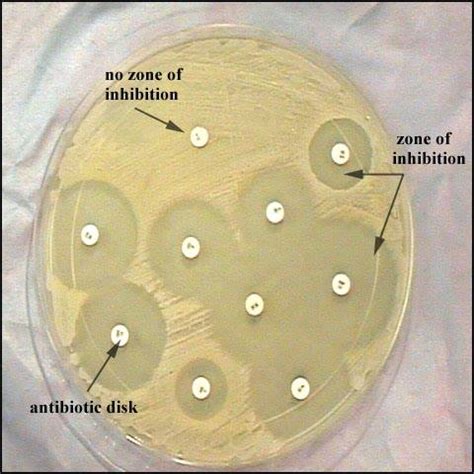
Table of Contents
Antimicrobial Sensitivity Testing: A Deep Dive into the Kirby-Bauer Method
The Kirby-Bauer method, also known as the disk diffusion test, is a cornerstone of antimicrobial susceptibility testing (AST). This widely used technique provides crucial information for guiding antibiotic therapy, informing infection control practices, and contributing to epidemiological surveillance of antimicrobial resistance. Understanding the principles, procedure, and interpretation of the Kirby-Bauer method is essential for anyone involved in microbiology, infectious disease management, or public health. This comprehensive guide will explore all aspects of this vital laboratory procedure.
Understanding the Principles of the Kirby-Bauer Method
The Kirby-Bauer method relies on the principle of diffusion. Antimicrobial disks containing known concentrations of various antibiotics are placed on an agar plate that has been inoculated with a standardized inoculum of the bacterial isolate being tested. As the antibiotics diffuse outwards from the disk, they create a concentration gradient in the agar. If the bacteria are susceptible to the antibiotic, they will be inhibited from growing within a certain radius around the disk, creating a zone of inhibition (ZOI). The size of this zone correlates with the susceptibility of the bacteria to the specific antibiotic.
Key Factors Influencing ZOI Size:
- Antibiotic concentration: Higher concentrations generally lead to larger ZOI.
- Antibiotic diffusion rate: Different antibiotics diffuse at varying rates through agar.
- Bacterial growth rate: Rapidly growing bacteria will produce larger ZOIs in a shorter time.
- Bacterial inoculum size: A larger inoculum will result in smaller ZOIs.
- Agar depth: The depth of the agar influences diffusion and subsequently, ZOI size. Variations in depth can lead to inaccurate results.
- Incubation temperature and time: Incubation conditions must be carefully controlled to ensure accurate results. Incorrect temperature or incubation time can significantly affect ZOI size.
The Importance of Standardization
The accuracy and reliability of the Kirby-Bauer method heavily depend on strict adherence to standardized protocols. These protocols are crucial for ensuring reproducibility and comparability of results across different laboratories. Key standardization aspects include:
- Inoculum preparation: The bacterial inoculum must be standardized to ensure a consistent number of bacterial cells on the agar plate. This is typically achieved by adjusting the turbidity of the bacterial suspension to match a 0.5 McFarland standard. This ensures that the concentration of bacteria is consistent across all tests.
- Agar type and depth: Mueller-Hinton agar is the standard medium used for Kirby-Bauer testing due to its consistent composition and suitable properties for antibiotic diffusion. The depth of the agar in the Petri dish must be precisely controlled (typically 4 mm) to ensure consistent diffusion rates.
- Disk dispensing: Antibiotic disks must be dispensed accurately and evenly onto the agar surface. Overlapping or uneven distribution of disks can affect ZOI measurements.
- Incubation conditions: Plates are incubated under controlled conditions of temperature (usually 35°C) and atmospheric conditions (typically aerobic) for a specified time (usually 16-18 hours). Consistency in incubation is crucial for accurate ZOI interpretation.
- Measurement of ZOI: ZOI diameter is measured in millimeters using a ruler or caliper. Measurements should be taken carefully and accurately to ensure reliable interpretation.
The Step-by-Step Procedure of the Kirby-Bauer Method
The Kirby-Bauer method involves several precise steps:
1. Preparation of the bacterial inoculum: A pure culture of the bacterial isolate is grown on an appropriate agar plate. A portion of the culture is then suspended in sterile broth to achieve a 0.5 McFarland standard turbidity. This ensures the proper bacterial concentration for testing.
2. Inoculation of the agar plates: The standardized bacterial suspension is evenly spread over the surface of Mueller-Hinton agar plates using a sterile cotton swab. This creates a uniform lawn of bacteria for consistent antibiotic diffusion.
3. Disk application: Sterile forceps are used to gently place antibiotic disks onto the inoculated agar surface. Ensure disks are evenly spaced and do not overlap. The disks should be pressed gently to ensure good contact with the agar surface. A sufficient number of disks are placed to cover the range of potential antibiotics for that specific bacterial isolate.
4. Incubation: The inoculated plates are inverted and incubated under controlled conditions (typically 35°C for 16-18 hours). This allows sufficient time for bacterial growth and antibiotic diffusion.
5. Measurement of zone of inhibition: After incubation, the diameter of the zones of inhibition surrounding each antibiotic disk is measured in millimeters. This measurement is crucial for determining the susceptibility of the bacteria to each antibiotic. Measurements should always be made along the diameter perpendicular to the direction of the disk placement.
Interpreting the Results: Understanding Susceptibility Categories
Once the ZOI diameters are measured, they are compared to interpretive standards published by the Clinical and Laboratory Standards Institute (CLSI) or similar organizations. These standards provide guidelines for classifying bacterial isolates as susceptible, intermediate, or resistant based on the size of the ZOI for each antibiotic.
-
Susceptible (S): The bacteria are inhibited by the antibiotic at the clinically achievable concentrations. Treatment with the antibiotic is likely to be effective.
-
Intermediate (I): The clinical outcome is uncertain. The antibiotic may be effective if high concentrations can be achieved at the site of infection or if the bacteria are highly susceptible to the antibiotic. This category usually indicates the need for further investigation.
-
Resistant (R): The bacteria are not inhibited by the antibiotic at clinically achievable concentrations. Treatment with that particular antibiotic is unlikely to be effective.
Factors Affecting Interpretation
Several factors can influence the interpretation of Kirby-Bauer results:
-
Variations in bacterial strain: Different strains of the same bacterial species may exhibit different levels of susceptibility to certain antibiotics.
-
Antibiotic degradation: Some antibiotics are less stable and may degrade over time, leading to smaller ZOIs and potentially inaccurate results.
-
Presence of enzymes: Certain bacterial enzymes can inactivate antibiotics, reducing the size of the ZOI and leading to a false interpretation of resistance.
-
Use of inappropriate media: Using media other than Mueller-Hinton agar can alter diffusion rates and affect ZOI interpretation.
-
Incorrect incubation conditions: Deviations from the standardized incubation conditions can significantly affect bacterial growth and antibiotic diffusion, leading to inaccuracies.
-
Errors in technique: Inconsistent inoculum preparation, incorrect disk placement, or inaccurate ZOI measurements can affect the results.
The Importance of Quality Control in Kirby-Bauer Testing
Quality control (QC) procedures are essential for ensuring the accuracy and reliability of Kirby-Bauer testing. QC involves the use of control strains with known susceptibility patterns to monitor the performance of the test system. These control strains are tested alongside patient isolates to detect potential problems with the media, reagents, or technique. Inconsistencies between the observed and expected results indicate a problem that needs to be addressed before patient samples are tested.
Advanced Applications and Limitations of the Kirby-Bauer Method
While the Kirby-Bauer method is a widely used and valuable tool, it has some limitations. It provides a qualitative assessment of antibiotic susceptibility, and it doesn't determine the Minimum Inhibitory Concentration (MIC). MIC is a quantitative measure of the lowest concentration of antibiotic that inhibits bacterial growth. For more precise measurements, other techniques like broth microdilution or Etest can be utilized.
Despite its limitations, the Kirby-Bauer method has numerous advantages. It's relatively simple, inexpensive, and widely accessible. It's a valuable tool in resource-limited settings for guiding antibiotic therapy. Additionally, advances in technology have enhanced the method, such as automated systems for inoculum preparation and ZOI measurement, further increasing accuracy and efficiency.
Conclusion: The Enduring Relevance of the Kirby-Bauer Method
The Kirby-Bauer disk diffusion method remains a cornerstone of antimicrobial susceptibility testing. Its simplicity, accessibility, and reliability make it an invaluable tool for guiding antibiotic therapy, informing infection control practices, and contributing to epidemiological surveillance of antimicrobial resistance. However, it's crucial to remember that accurate and reliable results depend on strict adherence to standardized protocols and implementation of rigorous quality control measures. While advanced techniques provide more quantitative data, the Kirby-Bauer method remains an indispensable part of the clinical microbiology laboratory's arsenal in the ongoing battle against antimicrobial resistance. Continuous improvement of the method and its integration with other techniques ensures its continued relevance in the fight against infectious diseases. Ongoing research into alternative and advanced AST methodologies alongside improved training and standardization further underscores the critical role of the Kirby-Bauer method in combating antimicrobial resistance globally.
Latest Posts
Latest Posts
-
Standard Free Energy Of Formation Table
Mar 20, 2025
-
R Showing All Entries As Singularity In Regression
Mar 20, 2025
-
Physical Or Chemical Change Ice Melting
Mar 20, 2025
-
A Tiny Heart Case Study Answers
Mar 20, 2025
-
What Is The Molar Mass Of Sodium Chloride
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Antimicrobial Sensitivity Testing Kirby Bauer Method . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
