What Is The Molar Mass Of Sodium Chloride
Muz Play
Mar 20, 2025 · 5 min read
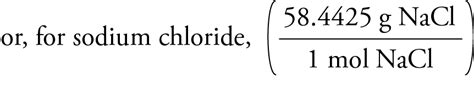
Table of Contents
What is the Molar Mass of Sodium Chloride? A Deep Dive into Atomic Mass, Molecular Weight, and Applications
The seemingly simple question, "What is the molar mass of sodium chloride?" opens a door to a fascinating exploration of chemistry's fundamental concepts. Understanding molar mass is crucial for various applications, from stoichiometric calculations in chemical reactions to determining concentrations in solutions. This article will not only answer the question directly but will delve into the underlying principles, calculations, and practical significance of sodium chloride's molar mass.
Understanding Atomic Mass and Molar Mass
Before we calculate the molar mass of sodium chloride (NaCl), let's clarify the key terms:
Atomic Mass
Each element possesses a unique atomic mass, representing the average mass of an atom of that element, taking into account the different isotopes and their relative abundances. Atomic mass is usually expressed in atomic mass units (amu), where 1 amu is approximately 1/12 the mass of a carbon-12 atom. You can find the atomic mass of elements on the periodic table. For example, the atomic mass of sodium (Na) is approximately 22.99 amu, and the atomic mass of chlorine (Cl) is approximately 35.45 amu. These values are averages reflecting the naturally occurring isotopic mix of each element.
Molar Mass
Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance containing as many elementary entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12. This number is Avogadro's number, approximately 6.022 x 10²³. The molar mass is numerically equivalent to the atomic mass (for elements) or molecular weight (for compounds) but expressed in grams per mole (g/mol).
Calculating the Molar Mass of Sodium Chloride (NaCl)
Sodium chloride is an ionic compound formed by the electrostatic attraction between sodium (Na⁺) cations and chloride (Cl⁻) anions. To calculate its molar mass, we simply add the atomic masses of its constituent elements:
- Atomic mass of Sodium (Na): 22.99 g/mol
- Atomic mass of Chlorine (Cl): 35.45 g/mol
Molar mass of NaCl = Atomic mass of Na + Atomic mass of Cl
Molar mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
Therefore, the molar mass of sodium chloride is 58.44 g/mol. This means that one mole of NaCl weighs 58.44 grams.
The Significance of Molar Mass in Chemical Calculations
The molar mass of NaCl is a cornerstone for various chemical calculations, including:
1. Stoichiometry
Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Knowing the molar mass of NaCl allows us to convert between mass, moles, and number of particles. For instance, if we have a reaction involving NaCl, we can use its molar mass to determine the number of moles of NaCl involved given a specific mass, or vice-versa. This is crucial for accurately predicting the yield of a reaction or determining the limiting reactant.
2. Solution Concentration
Molarity, a common unit of concentration, is defined as moles of solute per liter of solution. To prepare a solution of a specific molarity using NaCl, we need to know its molar mass to accurately weigh out the required amount of NaCl to dissolve in a given volume of solvent. For example, to prepare 1 liter of a 1M NaCl solution, we need to dissolve 58.44 grams of NaCl in enough water to make a total volume of 1 liter.
3. Determining Empirical and Molecular Formulas
Molar mass plays a critical role in determining the empirical and molecular formulas of compounds. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms of each element in a molecule. Knowing the molar mass of a compound along with its percentage composition allows us to determine both its empirical and molecular formulas.
4. Titrations
In acid-base titrations, molar mass is used to calculate the concentration of an unknown solution using a solution of known concentration. For example, if we titrate a solution of sodium hydroxide (NaOH) with a solution of hydrochloric acid (HCl), the molar mass of NaCl is indirectly used in the calculations as the reaction produces NaCl.
5. Other Applications
Molar mass has broader applications beyond simple chemical calculations. It is used in various fields, including:
- Material Science: Determining the molecular weight of polymers and other materials.
- Pharmaceuticals: Calculating drug dosages and formulations.
- Environmental Science: Analyzing the concentration of pollutants in water and air samples.
- Food Science: Determining the nutritional content of food products.
Isotopic Variations and Their Effect on Molar Mass
The atomic masses used in the calculation of NaCl's molar mass are average values. Chlorine, in particular, has two common isotopes, chlorine-35 and chlorine-37, with different natural abundances. The atomic mass of chlorine (35.45 amu) reflects this average. If we were working with a sample of NaCl enriched in one specific chlorine isotope, the molar mass would slightly differ. However, for most practical purposes, the average molar mass of 58.44 g/mol is sufficiently accurate.
Conclusion: Molar Mass - A Fundamental Concept in Chemistry
The molar mass of sodium chloride, 58.44 g/mol, is more than just a number; it's a fundamental concept bridging the microscopic world of atoms and molecules with the macroscopic world of laboratory measurements and chemical reactions. Its accurate calculation and application are essential for precise stoichiometric calculations, determining solution concentrations, and numerous other applications in various scientific and industrial disciplines. Understanding molar mass empowers us to perform accurate calculations, predict reaction outcomes, and gain a deeper understanding of the quantitative aspects of chemistry. This seemingly simple value is the cornerstone of much of our quantitative understanding of the chemical world.
Latest Posts
Latest Posts
-
Law Of Segregation Vs Independent Assortment
Mar 20, 2025
-
What Are The Functions Of A Root
Mar 20, 2025
-
How To Add Impedances In Parallel
Mar 20, 2025
-
Solving Linear Systems By Substitution Answer Key
Mar 20, 2025
-
Who Measured The Charge Of An Electron
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Sodium Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
