Who Measured The Charge Of An Electron
Muz Play
Mar 20, 2025 · 5 min read
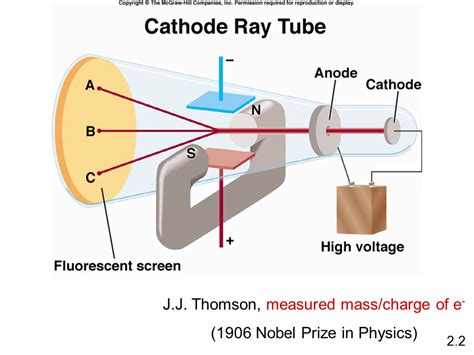
Table of Contents
Who Measured the Charge of an Electron? A Journey Through Scientific Discovery
The electron, a fundamental particle of matter carrying a negative charge, has captivated scientists for centuries. Understanding its properties, particularly its charge, has been pivotal in advancing our comprehension of the universe. Pinpointing who measured the charge of an electron, however, isn't as straightforward as it seems. It was a collaborative effort, a culmination of ingenious experiments and theoretical advancements built upon the work of many brilliant minds. This article delves into the fascinating history, highlighting the key players and their pivotal contributions to this monumental scientific achievement.
The Early Days: Suspecting the Existence of the Electron
Before we could measure the electron's charge, we needed to know it existed. This understanding arose from observations of cathode rays, luminous streams emanating from the cathode in a vacuum tube. Scientists like Julius Plücker and Johann Wilhelm Hittorf in the mid-19th century laid the groundwork, noting the rays' deflection by magnetic fields. Eugen Goldstein, in 1876, coined the term "cathode rays," furthering the investigation.
However, the nature of these rays remained a subject of intense debate. Were they waves or particles? Sir William Crookes, through meticulous experiments with cathode ray tubes, provided compelling evidence suggesting a particulate nature. His work, culminating in the development of the Crookes tube, paved the way for future discoveries.
J.J. Thomson: The Discovery of the Electron
The breakthrough came in 1897, with J.J. Thomson's groundbreaking experiments. Thomson, using refined cathode ray tubes, meticulously studied the deflection of cathode rays under the influence of both electric and magnetic fields. By carefully balancing these forces, he could determine the charge-to-mass ratio (e/m) of the particles comprising the rays. This ratio was significantly larger than that of any known ion, suggesting a far smaller, and therefore much lighter, particle.
Thomson's work didn't directly measure the charge e, but it provided crucial evidence for the existence of a subatomic particle—the electron. His discovery revolutionized our understanding of the atom, shattering the long-held belief in its indivisibility. It ushered in the era of subatomic physics and laid the foundation for future experiments aimed at determining the electron's charge independently.
Millikan's Oil Drop Experiment: A Landmark Achievement
While Thomson's work provided the charge-to-mass ratio, it was Robert Millikan, along with his student Harvey Fletcher, who directly measured the elementary electric charge using the famous oil drop experiment. This experiment, conducted between 1909 and 1913, is a cornerstone of physics education even today.
The Methodology: A Masterclass in Experimental Precision
Millikan's ingenious setup involved observing tiny oil droplets falling under gravity in a chamber. By applying an electric field, he could counteract the gravitational force and suspend the droplets. The key insight was that the oil droplets could acquire an electric charge through ionization of the air molecules.
By meticulously measuring the terminal velocity of the droplets both with and without the electric field, Millikan could determine the charge on each droplet. He found that the charges were always integer multiples of a fundamental value—a crucial piece of evidence indicating the existence of a fundamental unit of electric charge. This fundamental unit was indeed the charge of the electron.
Challenges and Refinements: Ensuring Accuracy
The oil drop experiment wasn't without its challenges. Factors like air viscosity, droplet evaporation, and electric field irregularities had to be carefully accounted for. Millikan and Fletcher's meticulous attention to detail and rigorous data analysis led to increasingly precise measurements. Their refined technique minimized systematic errors and ultimately yielded a value for the elementary charge consistent with modern measurements.
Controversy and Recognition: A Testament to Scientific Rigor
Despite its revolutionary impact, Millikan's work wasn't without controversy. Some critics questioned his data selection and analysis, suggesting a potential bias in favour of results supporting his hypothesis. However, later investigations and analysis have largely vindicated Millikan's findings. His persistent effort to refine his technique and meticulously account for potential errors is a testament to the scientific method's robustness.
His achievement earned him the 1923 Nobel Prize in Physics, a well-deserved recognition of his groundbreaking work.
Beyond Millikan: Further Refinements and Modern Measurements
While Millikan's oil drop experiment provided a landmark measurement, the quest for increasingly accurate values of the electron's charge has continued. Modern techniques employing sophisticated instrumentation have refined the measurement to an exceptional degree of precision.
These modern methods include:
- Quantum Hall effect: This phenomenon observed in two-dimensional electron systems at low temperatures and high magnetic fields provides a highly accurate determination of the fundamental constants, including the elementary charge.
- X-ray crystallography: By analyzing the diffraction patterns of X-rays interacting with crystals, scientists can deduce the charge distribution within the crystal lattice and obtain information about the elementary charge.
These advanced techniques confirm Millikan's findings and provide even more precise measurements of the electron's charge.
The Legacy: A Cornerstone of Modern Physics
The measurement of the electron's charge wasn't a singular event but a journey involving numerous scientists and their contributions. From the initial observations of cathode rays to the sophisticated techniques employed today, the story highlights the iterative nature of scientific progress.
J.J. Thomson's determination of the charge-to-mass ratio was pivotal in establishing the electron's existence. Robert Millikan's oil drop experiment, despite the controversies surrounding it, provided the first direct measurement of the elementary charge, a feat of experimental brilliance. Subsequent refinements and modern techniques have further enhanced our understanding of this fundamental constant.
The electron's charge, a fundamental constant in physics, plays a central role in countless phenomena, from atomic structure and chemical bonding to the behavior of materials and the operation of electronic devices. The scientific journey to measure this seemingly tiny quantity is a testament to human ingenuity and the power of collaborative scientific inquiry. It showcases how building upon previous work, meticulous experimentation, and rigorous data analysis can lead to profound advancements in our understanding of the universe. The precise measurement of the electron's charge remains a triumph of scientific achievement and a cornerstone of modern physics.
Latest Posts
Latest Posts
-
Law Of Segregation Vs Law Of Independent Assortment
Mar 20, 2025
-
Are Substances With A High Melting Point Soluble
Mar 20, 2025
-
Members Of The Kingdom Fungi Are Photosynthetic
Mar 20, 2025
-
Organic Chemistry Substitution And Elimination Reactions Practice Problems
Mar 20, 2025
-
What Is Gas To Solid Called
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Who Measured The Charge Of An Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
