Antimicrobial Sensitivity Testing The Kirby Bauer Method
Muz Play
Apr 01, 2025 · 6 min read
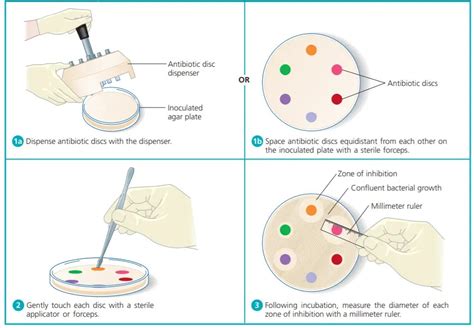
Table of Contents
Antimicrobial Sensitivity Testing: The Kirby-Bauer Method – A Comprehensive Guide
Antimicrobial sensitivity testing (AST) is a cornerstone of modern microbiology, providing crucial information for guiding treatment decisions in bacterial infections. Among the various AST methods available, the Kirby-Bauer disk diffusion test, also known as the Bauer-Kirby method, remains a widely used and reliable technique for determining the susceptibility of bacterial isolates to different antimicrobial agents. This comprehensive guide delves into the intricacies of the Kirby-Bauer method, covering its principles, procedure, interpretation, limitations, and its ongoing relevance in the fight against antimicrobial resistance.
Understanding the Principles of the Kirby-Bauer Method
The Kirby-Bauer method relies on the principle of diffusion. Antimicrobial disks containing a known concentration of a specific antibiotic are placed onto a bacterial lawn grown on a standardized agar plate. The antibiotic diffuses radially outwards from the disk, creating a concentration gradient. If the bacterium is susceptible to the antibiotic, it will be inhibited from growing in the area surrounding the disk, resulting in a zone of inhibition. The diameter of this zone is then measured and compared to established interpretive standards to determine the susceptibility of the bacterium to that particular antibiotic.
Key Factors Influencing Zone Diameter
Several crucial factors influence the size of the zone of inhibition and, therefore, the interpretation of the test results:
- Antibiotic concentration: Disks contain a standardized concentration of the antibiotic. Variations in this concentration can significantly affect the zone size.
- Diffusion rate of the antibiotic: Each antibiotic diffuses through the agar at a different rate, influencing the zone size.
- Bacterial inoculum size: The number of bacteria initially seeded onto the agar plate affects the clarity and size of the zone of inhibition. Too many bacteria can mask the inhibition effect.
- Growth rate of the bacteria: Faster-growing bacteria may show slightly larger zones of inhibition.
- Agar depth: The standard depth of the agar is crucial as it affects the diffusion rate. Variations can lead to misinterpretations.
- Incubation temperature and time: These parameters are standardized to ensure consistent results. Deviations can impact bacterial growth and zone size.
The Step-by-Step Procedure of the Kirby-Bauer Method
The Kirby-Bauer test follows a meticulous protocol to ensure accuracy and reproducibility. Here's a detailed step-by-step guide:
1. Preparation of Bacterial Inoculum
A pure bacterial culture is needed. A standardized inoculum is prepared by adjusting the turbidity of a bacterial suspension to match a 0.5 McFarland standard. This ensures a consistent bacterial load across all tests.
2. Preparation of the Mueller-Hinton Agar Plate
Mueller-Hinton agar (MHA) is the standard medium used due to its consistent composition and suitability for antibiotic diffusion. The agar is poured into standardized Petri dishes to a specific depth (typically 4 mm). Proper depth is crucial to prevent skewed results.
3. Inoculation of the Agar Plate
The standardized bacterial inoculum is evenly spread across the surface of the MHA plate using a sterile cotton swab. This creates a uniform bacterial lawn. It’s crucial to avoid excessive or inadequate inoculation.
4. Application of Antimicrobial Disks
Antimicrobial disks containing pre-defined concentrations of various antibiotics are carefully placed onto the inoculated agar plate using sterile forceps. The disks should be evenly spaced and pressed gently to ensure good contact with the agar.
5. Incubation
The inoculated plates are incubated under controlled conditions (usually at 35°C for 16-18 hours) in an aerobic environment. Consistent temperature and incubation time are paramount for accurate interpretation.
6. Measurement and Interpretation of Zone Diameters
After incubation, the diameter of the zone of inhibition around each disk is measured in millimeters using a ruler. These measurements are then compared to established interpretive standards provided by the Clinical and Laboratory Standards Institute (CLSI) or similar organizations. These standards define zones of inhibition as susceptible, intermediate, or resistant, providing guidance for treatment decisions.
Interpreting the Results: Susceptible, Intermediate, and Resistant
The interpretation of the zone diameters is crucial and depends on the specific antibiotic and the bacterium being tested. CLSI guidelines provide tables that correlate the zone diameter with the susceptibility category:
- Susceptible (S): The bacterium is inhibited by the usually achievable concentrations of the antibiotic in vivo. Treatment with this antibiotic is likely to be successful.
- Intermediate (I): The results are uncertain. The antibiotic concentration may be achievable at the infection site, but higher doses or prolonged treatment might be required. Further investigation may be necessary.
- Resistant (R): The antibiotic is unlikely to be effective at inhibiting the growth of the bacterium, even at high concentrations. Alternative treatment options should be explored.
Limitations of the Kirby-Bauer Method
While the Kirby-Bauer method is a valuable tool, it has certain limitations:
- It does not provide Minimum Inhibitory Concentration (MIC): The Kirby-Bauer method only provides qualitative data (susceptible, intermediate, resistant), not the quantitative MIC value, which is the lowest antibiotic concentration that inhibits bacterial growth. MIC values are crucial for optimal dosing and minimizing adverse effects.
- Not suitable for all bacteria or antibiotics: Some fastidious bacteria or antibiotics may not be amenable to this method.
- Potential for error: Technical errors in any step of the procedure can significantly affect the results. Strict adherence to standardized protocols is essential.
- Does not detect synergistic or antagonistic effects: The method assesses the effect of individual antibiotics only and doesn't evaluate the combined effect of multiple drugs.
- Limited predictive power for in vivo efficacy: The in vitro results may not always perfectly predict the in vivo outcome, due to factors like pharmacokinetic and pharmacodynamic properties of the antibiotics and host immune response.
The Kirby-Bauer Method in the Era of Antimicrobial Resistance
The rise of antimicrobial resistance (AMR) poses a significant global health threat. The Kirby-Bauer method plays a crucial role in combating AMR by:
- Guiding empirical therapy: Providing rapid and cost-effective susceptibility data that informs initial treatment decisions.
- Monitoring resistance patterns: Tracking changes in bacterial susceptibility over time to understand the spread of resistance.
- Supporting infection control measures: Identifying resistant pathogens in healthcare settings to guide infection prevention and control strategies.
- Facilitating research on new antimicrobial agents: Providing a platform for evaluating the effectiveness of novel antibiotics.
Advances and Modifications of the Kirby-Bauer Method
While the basic principles remain unchanged, several modifications and technological advancements have enhanced the Kirby-Bauer method:
- Automated systems: Automated systems are available to enhance speed and reduce human error in inoculum preparation, disk placement, and zone size measurement.
- Digital image analysis: Software-assisted image analysis provides more objective and precise measurement of zone diameters.
- Combination of Kirby-Bauer with other techniques: The Kirby-Bauer method can be used in conjunction with other techniques like E-test or broth microdilution to determine MIC values.
Conclusion: The Enduring Importance of the Kirby-Bauer Method
The Kirby-Bauer disk diffusion test remains an indispensable tool in clinical microbiology laboratories worldwide. Despite its limitations, its simplicity, affordability, and reliability make it an essential method for determining bacterial susceptibility to antimicrobial agents. It continues to play a vital role in the fight against antimicrobial resistance, guiding treatment decisions, and informing infection control strategies. As we face the growing challenge of AMR, the importance of accurate and efficient AST methods like the Kirby-Bauer method will only continue to grow, alongside its ongoing refinements and adaptations. The future of antimicrobial stewardship relies heavily on the continued use and improvement of techniques like this to ensure effective treatment and preservation of existing antibiotics.
Latest Posts
Latest Posts
-
Diagram The Life Cycle Of A Liverwort
May 09, 2025
-
Law Of Conservation Of Mass States That
May 09, 2025
-
Chloroplasts And Cell Walls Are Found Only In
May 09, 2025
-
If A Number Is Real Then It Is Also Rational
May 09, 2025
-
Electron Volt Is The Unit Of
May 09, 2025
Related Post
Thank you for visiting our website which covers about Antimicrobial Sensitivity Testing The Kirby Bauer Method . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.