Ap Chemistry Strong Acids And Bases
Muz Play
Mar 16, 2025 · 6 min read
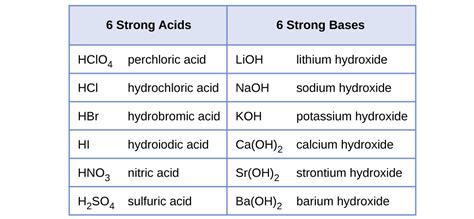
Table of Contents
AP Chemistry: Mastering Strong Acids and Bases
Strong acids and bases are fundamental concepts in AP Chemistry, forming the bedrock for understanding numerous chemical reactions and equilibrium systems. This comprehensive guide will delve into the intricacies of strong acids and bases, exploring their properties, reactions, calculations, and applications. We'll cover everything you need to ace this crucial section of your AP Chemistry exam.
Understanding Strong Acids
A strong acid is defined as an acid that completely dissociates into its ions when dissolved in water. This means that 100% of the acid molecules break apart into hydrogen ions (H⁺, often represented as hydronium ions, H₃O⁺) and their conjugate bases. This complete dissociation is the key characteristic that distinguishes strong acids from weak acids, which only partially dissociate.
Key Properties of Strong Acids:
- Complete Dissociation: This is the defining property. In aqueous solution, strong acids essentially exist entirely as ions.
- High Acidity: They exhibit significantly high acidity, measured by their low pH values (typically below 3).
- Predictable Reactions: Their reactions are usually straightforward and easy to predict due to their complete dissociation.
- Strong Conjugate Bases: The conjugate bases of strong acids are very weak, meaning they have negligible tendency to react with water and reform the acid.
Common Strong Acids:
It's crucial to memorize the common strong acids. While the list can vary slightly depending on the source, these are generally considered the standard strong acids:
- Hydrochloric acid (HCl): A common laboratory reagent and a component of gastric acid.
- Hydrobromic acid (HBr): Similar in properties to HCl.
- Hydroiodic acid (HI): Also similar in properties to HCl and HBr.
- Nitric acid (HNO₃): A powerful oxidizing agent and widely used in various industrial processes.
- Sulfuric acid (H₂SO₄): A diprotic acid (donates two protons) and a crucial industrial chemical. Note that only its first proton dissociation is considered completely strong; the second dissociation is weaker.
- Perchloric acid (HClO₄): One of the strongest known acids.
Note: Other acids, while highly reactive, might not undergo complete dissociation under all conditions and thus are not technically considered strong acids.
Calculating pH of Strong Acid Solutions:
Calculating the pH of a strong acid solution is relatively straightforward because of the complete dissociation. The concentration of H⁺ ions is equal to the initial concentration of the acid (assuming complete dissociation).
Formula: pH = -log₁₀[H⁺]
Where [H⁺] is the concentration of hydrogen ions in moles per liter (M).
Example: What is the pH of a 0.1 M solution of HCl?
Since HCl is a strong acid, it completely dissociates: HCl → H⁺ + Cl⁻. Therefore, [H⁺] = 0.1 M.
pH = -log₁₀(0.1) = 1
Understanding Strong Bases
A strong base is defined as a base that completely dissociates into its ions when dissolved in water. This means that 100% of the base molecules break apart into hydroxide ions (OH⁻) and their conjugate acids. This complete dissociation is the defining characteristic that differentiates strong bases from weak bases, which only partially dissociate.
Key Properties of Strong Bases:
- Complete Dissociation: As with strong acids, complete dissociation is the defining property.
- High Basicity: They exhibit high basicity, measured by their high pH values (typically above 11).
- Predictable Reactions: Their reactions are generally predictable due to their complete dissociation.
- Strong Conjugate Acids: The conjugate acids of strong bases are very weak.
Common Strong Bases:
The most common strong bases are the alkali metal hydroxides (Group 1A) and the heavier alkaline earth metal hydroxides (Group 2A).
- Sodium hydroxide (NaOH): Also known as lye or caustic soda; widely used in many industrial and household applications.
- Potassium hydroxide (KOH): Similar in properties to NaOH.
- Lithium hydroxide (LiOH): Used in various applications, including in batteries.
- Calcium hydroxide (Ca(OH)₂): Also known as slaked lime; less soluble than the Group 1A hydroxides.
- Barium hydroxide (Ba(OH)₂): Relatively more soluble than calcium hydroxide.
Note: While other bases exist, these are typically the ones categorized as strong bases in AP Chemistry. The solubility of the base in water significantly affects its strength as a base in solution; an insoluble base will not show strong base behavior even though its inherent dissociation might be high.
Calculating pOH and pH of Strong Base Solutions:
For strong bases, the concentration of OH⁻ ions is equal to the initial concentration of the base (assuming complete dissociation and high solubility). We can then use the following equations:
Formula: pOH = -log₁₀[OH⁻]
Since pH + pOH = 14 at 25°C, we can then calculate the pH.
Example: What is the pH of a 0.01 M solution of NaOH?
Since NaOH is a strong base, it completely dissociates: NaOH → Na⁺ + OH⁻. Therefore, [OH⁻] = 0.01 M.
pOH = -log₁₀(0.01) = 2
pH = 14 - pOH = 14 - 2 = 12
Neutralization Reactions: Strong Acid-Strong Base Titrations
A crucial application of strong acids and bases is in neutralization reactions. When a strong acid and a strong base react, they neutralize each other, producing water and a salt. This reaction is highly exothermic (releases heat).
General Equation: Strong Acid + Strong Base → Water + Salt
Example: HCl(aq) + NaOH(aq) → H₂O(l) + NaCl(aq)
Titrations: Titration is a common laboratory technique used to determine the concentration of an unknown solution (analyte) using a solution of known concentration (titrant). Strong acid-strong base titrations are particularly straightforward due to the complete dissociation of both the acid and the base. The equivalence point, where the moles of acid equal the moles of base, is easily determined using an indicator like phenolphthalein.
Calculations: Stoichiometry plays a vital role in titrations. The balanced chemical equation is used to relate the moles of acid to the moles of base at the equivalence point.
Example: 25.00 mL of an unknown concentration of HCl is titrated with 0.100 M NaOH. The equivalence point is reached after 20.00 mL of NaOH is added. What is the concentration of the HCl solution?
Moles of NaOH = (0.100 mol/L) * (0.0200 L) = 0.00200 mol
From the balanced equation (HCl + NaOH → H₂O + NaCl), the mole ratio of HCl to NaOH is 1:1. Therefore, moles of HCl = 0.00200 mol.
Concentration of HCl = (0.00200 mol) / (0.02500 L) = 0.0800 M
Applications of Strong Acids and Bases
Strong acids and bases have numerous applications across various fields:
- Industrial Processes: Sulfuric acid is a cornerstone of the chemical industry, used in fertilizer production, petroleum refining, and metal processing. NaOH is used in soap and paper manufacturing.
- Laboratory Reagents: HCl and NaOH are common laboratory reagents used in various chemical analyses and syntheses.
- Cleaning Products: Strong bases are components in many drain cleaners and other cleaning agents.
- Food and Beverage Industry: Controlled use of acids and bases is crucial in food processing and preservation.
- Medicine: Hydrochloric acid is a component of gastric acid aiding in digestion; controlled use of bases is crucial in pharmaceutical production.
Advanced Topics and Considerations:
- Ionic Strength and Activity Coefficients: At higher concentrations, the complete dissociation assumption becomes less accurate due to ion-ion interactions. Activity coefficients are used to correct for these deviations from ideal behavior.
- Temperature Effects: The dissociation constants of acids and bases are temperature-dependent.
- Non-Aqueous Solvents: The behavior of acids and bases can differ significantly in non-aqueous solvents compared to water.
Conclusion:
Mastering the concepts of strong acids and bases is essential for success in AP Chemistry. Understanding their properties, reactions, and applications, along with mastering the related calculations, will significantly improve your understanding of chemical equilibrium, titrations, and a wide range of chemical processes. Remember to practice numerous problems to reinforce your understanding and build confidence for the AP exam. By focusing on the core concepts and working through practice problems, you'll be well-prepared to tackle this important topic.
Latest Posts
Latest Posts
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Ap Chemistry Strong Acids And Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
