Differential Rate Law For Zero Order Reaction
Muz Play
Mar 17, 2025 · 7 min read
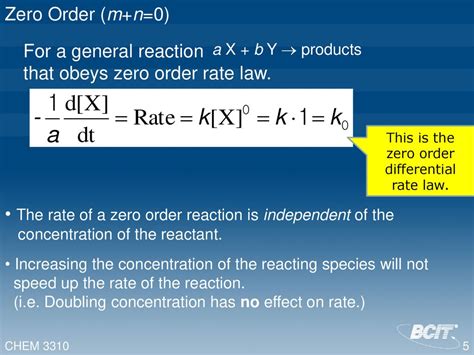
Table of Contents
Differential Rate Law for Zero-Order Reactions: A Comprehensive Guide
Understanding reaction kinetics is crucial in chemistry, allowing us to predict reaction rates and optimize processes. One fundamental aspect of reaction kinetics is the differential rate law, which describes the instantaneous rate of a reaction as a function of reactant concentrations. This article will delve into the differential rate law specifically for zero-order reactions, providing a comprehensive understanding of their characteristics, derivation, and applications.
What is a Zero-Order Reaction?
A zero-order reaction is a chemical reaction where the rate of the reaction is independent of the concentration of the reactants. This might seem counterintuitive at first, but it arises from specific reaction mechanisms, often involving a rate-limiting step that is not directly dependent on reactant concentration. The rate remains constant throughout the reaction until one of the reactants is completely consumed.
Key Characteristics of Zero-Order Reactions:
- Constant Rate: The most defining feature is the constant rate, regardless of reactant concentration.
- Rate = k: The rate of the reaction is solely determined by the rate constant, k.
- Linear Concentration vs. Time Plot: Plotting the concentration of the reactant versus time yields a straight line with a negative slope equal to -k.
- Half-life Dependence on Initial Concentration: Unlike first and second-order reactions, the half-life of a zero-order reaction is dependent on the initial concentration of the reactant.
Understanding the Differential Rate Law
The differential rate law, also known as the rate law, expresses the rate of a reaction in terms of the instantaneous change in concentration of reactants over time. For a generic reaction:
aA + bB → products
The general differential rate law is:
Rate = k[A]<sup>m</sup>[B]<sup>n</sup>
Where:
- Rate: The instantaneous rate of the reaction.
- k: The rate constant (a temperature-dependent constant).
- [A] and [B]: The concentrations of reactants A and B.
- m and n: The orders of the reaction with respect to reactants A and B (determined experimentally).
For a zero-order reaction, the exponents m and n for all reactants are zero. Therefore, the differential rate law simplifies to:
Rate = k
This means the rate of the reaction is simply equal to the rate constant, regardless of the concentrations of the reactants.
Derivation of the Integrated Rate Law
While the differential rate law provides the instantaneous rate, the integrated rate law allows us to calculate the concentration of a reactant at any time during the reaction. For a zero-order reaction, the derivation of the integrated rate law starts with the differential rate law:
d[A]/dt = -k
This equation states that the change in concentration of reactant A with respect to time is constant and equal to -k. To obtain the integrated rate law, we integrate both sides with respect to time:
∫d[A] = -k∫dt
Integrating gives:
[A]<sub>t</sub> - [A]<sub>0</sub> = -kt
Rearranging the equation, we arrive at the integrated rate law for a zero-order reaction:
[A]<sub>t</sub> = [A]<sub>0</sub> - kt
Where:
- [A]<sub>t</sub>: The concentration of reactant A at time t.
- [A]<sub>0</sub>: The initial concentration of reactant A at time t = 0.
- k: The rate constant.
- t: The reaction time.
This equation shows a linear relationship between concentration and time, forming the basis for determining the rate constant and predicting reactant concentration at any time.
Determining the Rate Constant (k)
The integrated rate law ([A]<sub>t</sub> = [A]<sub>0</sub> - kt) provides a straightforward method for determining the rate constant, k, of a zero-order reaction. By plotting the concentration of the reactant ([A]<sub>t</sub>) versus time (t), a straight line is obtained. The slope of this line is equal to -k. Therefore, the magnitude of the slope provides the value of the rate constant.
Half-life of a Zero-Order Reaction
The half-life (t<sub>1/2</sub>) is the time required for the concentration of a reactant to decrease to half its initial value. For a zero-order reaction, we can determine the half-life by substituting [A]<sub>t</sub> = [A]<sub>0</sub>/2 into the integrated rate law:
[A]<sub>0</sub>/2 = [A]<sub>0</sub> - kt<sub>1/2</sub>
Solving for t<sub>1/2</sub> gives:
t<sub>1/2</sub> = [A]<sub>0</sub> / 2k
This equation clearly shows that the half-life of a zero-order reaction is directly proportional to the initial concentration of the reactant ([A]<sub>0</sub>) and inversely proportional to the rate constant (k). Unlike first and second-order reactions, the half-life is not constant but depends on the initial concentration.
Examples of Zero-Order Reactions
Although less common than first or second-order reactions, several processes exhibit zero-order kinetics under specific conditions. Here are some examples:
- Enzyme-catalyzed reactions at high substrate concentrations: When the concentration of the substrate is significantly higher than the concentration of the enzyme, the enzyme becomes saturated. Further increases in substrate concentration do not increase the reaction rate, resulting in zero-order kinetics. This is because all enzyme active sites are occupied, and the rate is limited by the enzyme's turnover number.
- Photochemical reactions: In photochemical reactions, the rate is often determined by the intensity of the light source rather than the reactant concentration. If light intensity is constant, the reaction will proceed at a constant rate, irrespective of reactant concentration, demonstrating zero-order kinetics.
- Gas-phase reactions on a catalyst surface: When a gas-phase reaction occurs on the surface of a catalyst, the rate might become independent of the gas concentration if the catalyst surface becomes saturated with reactant molecules.
- Certain heterogeneous catalytic reactions: These reactions involve reactants in different phases (e.g., gas-solid), where the reaction rate is controlled by the surface area available for reaction rather than the bulk concentration of the reactants.
Applications of Zero-Order Kinetics
The understanding of zero-order kinetics has various applications in different fields:
- Pharmacokinetics: Zero-order kinetics is important in understanding drug absorption and elimination. Certain drugs are eliminated from the body at a constant rate, independent of their concentration, following zero-order kinetics. This information is crucial for determining appropriate dosage regimens.
- Environmental Chemistry: Zero-order kinetics can be used to model the degradation of pollutants in the environment under certain conditions. This helps in predicting the persistence of pollutants and developing effective remediation strategies.
- Industrial Processes: In industrial chemical processes, understanding zero-order kinetics can be beneficial in optimizing reaction conditions and controlling reaction rates. This can lead to improved efficiency and product yield.
- Analytical Chemistry: The principles of zero-order kinetics are used in various analytical techniques, allowing scientists to analyze reaction rates and determine the concentrations of reactants and products.
Distinguishing Zero-Order Reactions from Other Orders
It's crucial to distinguish zero-order reactions from first and second-order reactions. This is typically done by analyzing the concentration-time data:
- Zero-order: A plot of [A] vs. t gives a straight line with a slope of -k.
- First-order: A plot of ln[A] vs. t gives a straight line with a slope of -k.
- Second-order: A plot of 1/[A] vs. t gives a straight line with a slope of k.
By analyzing the linearity of these plots, one can determine the order of the reaction and consequently determine the rate constant.
Conclusion
Zero-order reactions, though less prevalent than other orders, represent an important class of reactions with unique characteristics and applications. Understanding the differential and integrated rate laws for zero-order reactions is essential for accurately predicting reaction rates, determining reaction mechanisms, and optimizing various chemical and industrial processes. The constant rate, linear concentration-time plot, and half-life dependence on initial concentration are key features that distinguish zero-order reactions from others, enabling accurate characterization and application in diverse scientific and technological fields. This comprehensive understanding allows for better control and prediction within reaction systems, leading to more efficient and effective processes across various disciplines.
Latest Posts
Latest Posts
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Differential Rate Law For Zero Order Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
