Are Acids Proton Acceptors Or Donors
Muz Play
Mar 18, 2025 · 6 min read
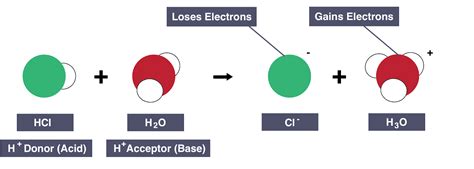
Table of Contents
Are Acids Proton Acceptors or Donors? Understanding the Brønsted-Lowry Definition
The question, "Are acids proton acceptors or donors?" is a fundamental concept in chemistry, crucial for understanding acid-base reactions. The short answer is: acids are proton donors. This definition stems from the Brønsted-Lowry theory, a widely accepted model for explaining acid-base behavior. Let's delve deeper into this definition, exploring its implications and contrasting it with other acid-base theories.
The Brønsted-Lowry Definition: The Heart of the Matter
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, defines acids and bases based on their ability to donate or accept protons (H⁺ ions). According to this theory:
-
An acid is a substance that donates a proton (H⁺) to another substance. It's a proton donor. Think of it as something that's willing to give up a hydrogen ion.
-
A base is a substance that accepts a proton (H⁺) from another substance. It's a proton acceptor. It's looking to gain a hydrogen ion.
This definition significantly expands the scope of acid-base reactions beyond the limitations of the Arrhenius theory, which restricts acids to substances that produce H⁺ ions in aqueous solutions and bases to substances that produce OH⁻ ions. The Brønsted-Lowry theory encompasses a broader range of reactions, including those that don't involve water.
Understanding Proton Donation: A Closer Look
When an acid donates a proton, it undergoes a chemical transformation. This process is often depicted using chemical equations. For example, consider the reaction between hydrochloric acid (HCl) and water:
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
In this reaction:
- HCl acts as an acid, donating a proton (H⁺) to a water molecule.
- H₂O acts as a base, accepting the proton from HCl.
- H₃O⁺ (hydronium ion) is formed, representing the protonated water molecule.
- Cl⁻ (chloride ion) is the conjugate base of HCl, the species remaining after the acid donates its proton.
This reaction demonstrates the essence of the Brønsted-Lowry definition: acids donate protons, and bases accept them. The reaction is an equilibrium, meaning it can proceed in both directions. The hydronium ion can donate a proton back to the chloride ion, reforming HCl and water.
Conjugate Acid-Base Pairs: A Dynamic Duo
The Brønsted-Lowry theory introduces the concept of conjugate acid-base pairs. In an acid-base reaction, the acid donates a proton to form its conjugate base, and the base accepts a proton to form its conjugate acid. In the HCl and water example:
- HCl (acid) and Cl⁻ (conjugate base) form a conjugate pair.
- H₂O (base) and H₃O⁺ (conjugate acid) form another conjugate pair.
Understanding conjugate pairs is crucial for analyzing acid-base reactions and predicting their equilibrium positions. A strong acid will have a weak conjugate base, and vice-versa. A weak acid will have a relatively stronger conjugate base.
Beyond Water: Expanding the Scope of Acid-Base Reactions
The beauty of the Brønsted-Lowry theory lies in its versatility. It isn't restricted to reactions involving water. Consider the reaction between ammonia (NH₃) and hydrogen chloride (HCl) in the gas phase:
NH₃(g) + HCl(g) → NH₄⁺(g) + Cl⁻(g)
Here:
- HCl acts as the acid, donating a proton.
- NH₃ acts as the base, accepting a proton.
- NH₄⁺ (ammonium ion) is the conjugate acid of ammonia.
- Cl⁻ (chloride ion) is the conjugate base of HCl.
This reaction exemplifies the broader applicability of the Brønsted-Lowry theory. The reaction takes place without the involvement of water, highlighting that acid-base reactions are not limited to aqueous solutions.
Comparing Brønsted-Lowry with Other Acid-Base Theories
While the Brønsted-Lowry theory is widely used, it's important to understand its relationship to other acid-base theories:
1. Arrhenius Theory: This older theory defines acids as substances that produce H⁺ ions in water and bases as substances that produce OH⁻ ions in water. While simpler, it is limited by its reliance on aqueous solutions. The Brønsted-Lowry theory is a more general and encompassing theory.
2. Lewis Theory: This theory offers the broadest definition of acids and bases. A Lewis acid is defined as an electron-pair acceptor, and a Lewis base is an electron-pair donor. While seemingly unrelated to protons at first glance, the Brønsted-Lowry definition is actually a subset of the Lewis definition. Proton donation involves the transfer of a proton, which is an electron-pair acceptor (Lewis acid). Therefore, every Brønsted-Lowry acid is also a Lewis acid. However, not all Lewis acids are Brønsted-Lowry acids (e.g., BF₃ can accept an electron pair without donating a proton).
Examples of Brønsted-Lowry Acids and Bases
Let's explore several common examples to solidify our understanding:
Strong Acids (completely dissociate in water):
- Hydrochloric acid (HCl): HCl → H⁺ + Cl⁻
- Sulfuric acid (H₂SO₄): H₂SO₄ → 2H⁺ + SO₄²⁻
- Nitric acid (HNO₃): HNO₃ → H⁺ + NO₃⁻
Weak Acids (partially dissociate in water):
- Acetic acid (CH₃COOH): CH₃COOH ⇌ H⁺ + CH₃COO⁻
- Carbonic acid (H₂CO₃): H₂CO₃ ⇌ H⁺ + HCO₃⁻
- Phosphoric acid (H₃PO₄): H₃PO₄ ⇌ H⁺ + H₂PO₄⁻
Strong Bases (completely dissociate in water):
- Sodium hydroxide (NaOH): NaOH → Na⁺ + OH⁻
- Potassium hydroxide (KOH): KOH → K⁺ + OH⁻
- Calcium hydroxide (Ca(OH)₂): Ca(OH)₂ → Ca²⁺ + 2OH⁻
Weak Bases (partially dissociate in water):
- Ammonia (NH₃): NH₃ + H₂O ⇌ NH₄⁺ + OH⁻
- Methylamine (CH₃NH₂): CH₃NH₂ + H₂O ⇌ CH₃NH₃⁺ + OH⁻
- Pyridine (C₅H₅N): C₅H₅N + H₂O ⇌ C₅H₅NH⁺ + OH⁻
Applications of the Brønsted-Lowry Theory
The Brønsted-Lowry theory has far-reaching applications in various fields:
-
Analytical Chemistry: Titration, a common analytical technique for determining the concentration of a substance, relies heavily on acid-base reactions governed by the Brønsted-Lowry theory.
-
Biochemistry: Many biological processes involve acid-base reactions, such as enzyme catalysis and protein folding. Understanding proton donation and acceptance is crucial for comprehending these processes.
-
Environmental Science: Acid rain, a significant environmental problem, is caused by the release of acidic gases into the atmosphere. The Brønsted-Lowry theory is essential for understanding the chemical reactions involved.
-
Industrial Chemistry: Many industrial processes involve acid-base catalysis, where acids or bases are used to speed up chemical reactions. Knowing which substances act as proton donors or acceptors is crucial for optimizing these processes.
Conclusion: A Fundamental Concept in Chemistry
The Brønsted-Lowry definition of acids as proton donors is a cornerstone of modern chemistry. Its elegance and broad applicability make it a crucial concept for understanding a wide range of chemical phenomena. By grasping the principles of proton donation and acceptance, one can better comprehend acid-base reactions, their equilibrium, and their importance across various scientific disciplines. Remember, while the Arrhenius and Lewis theories offer different perspectives, the Brønsted-Lowry theory remains a vital framework for interpreting and predicting the behavior of acids and bases in countless chemical interactions. Further exploration of this topic will undoubtedly enhance your understanding of chemistry's fundamental principles.
Latest Posts
Latest Posts
-
An Intermediate Phenotype Indicates That A Trait Has Dominance
Mar 19, 2025
-
Plant Is Where Photosynthesis Takes Place
Mar 19, 2025
-
The Force Driving Plate Tectonics Is
Mar 19, 2025
-
Converting Double Integrals To Polar Coordinates
Mar 19, 2025
-
How Do You Convert Moles To Volume
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Are Acids Proton Acceptors Or Donors . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
