How Do You Convert Moles To Volume
Muz Play
Mar 19, 2025 · 5 min read
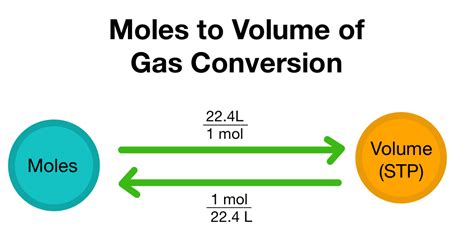
Table of Contents
How Do You Convert Moles to Volume? A Comprehensive Guide
Converting moles to volume is a fundamental concept in chemistry, crucial for various calculations and experiments. Understanding this conversion relies heavily on the ideal gas law and the molar volume of substances. This comprehensive guide will walk you through the process, explaining the underlying principles, providing step-by-step instructions, and offering examples to solidify your understanding.
Understanding the Relationship Between Moles and Volume
The connection between moles and volume isn't direct; it depends on the state of the substance. For gases, we use the ideal gas law. For liquids and solids, we need the substance's density or molar volume. Let's explore each case separately.
Converting Moles to Volume of Gases: The Ideal Gas Law
The ideal gas law, PV = nRT, is the cornerstone of this conversion for gases. Let's break down each variable:
- P: Pressure (usually in atmospheres, atm)
- V: Volume (usually in liters, L) – This is what we're solving for.
- n: Number of moles (mol) – This is our starting point.
- R: Ideal gas constant (0.0821 L·atm/mol·K)
- T: Temperature (in Kelvin, K)
To convert moles to volume, we rearrange the ideal gas law to solve for V:
V = nRT / P
Example 1: Calculating the Volume of a Gas
Let's say we have 2 moles of an ideal gas at a pressure of 1 atm and a temperature of 273.15 K (0°C). What's the volume?
-
Identify your known variables:
- n = 2 mol
- R = 0.0821 L·atm/mol·K
- T = 273.15 K
- P = 1 atm
-
Apply the formula:
V = (2 mol * 0.0821 L·atm/mol·K * 273.15 K) / 1 atm
-
Calculate:
V ≈ 44.8 L
Therefore, 2 moles of this ideal gas occupy approximately 44.8 liters under these conditions.
Considering Non-Ideal Gases
It's important to note that the ideal gas law is an approximation. Real gases deviate from ideal behavior, especially at high pressures and low temperatures. For more accurate calculations involving real gases, you'd need to use more complex equations of state, such as the van der Waals equation. However, for many introductory chemistry problems, the ideal gas law provides a reasonable approximation.
Converting Moles to Volume of Liquids and Solids: Density and Molar Volume
Liquids and solids are much denser than gases, and their volumes are less sensitive to changes in temperature and pressure. To convert moles to volume for liquids and solids, we use either the substance's density or its molar volume.
Using Density:
Density (ρ) is mass (m) per unit volume (V): ρ = m/V. We can rearrange this to solve for volume: V = m/ρ. The mass (m) can be calculated from the number of moles (n) and the molar mass (M) of the substance: m = nM.
Therefore, the formula for converting moles to volume using density is:
V = nM/ρ
Where:
- V = volume
- n = number of moles
- M = molar mass (g/mol)
- ρ = density (g/mL or g/cm³)
Example 2: Calculating the Volume of a Liquid
Let's say we have 1 mole of ethanol (C₂H₅OH). The molar mass of ethanol is approximately 46 g/mol, and its density is approximately 0.789 g/mL. What's the volume?
-
Identify your known variables:
- n = 1 mol
- M = 46 g/mol
- ρ = 0.789 g/mL
-
Apply the formula:
V = (1 mol * 46 g/mol) / 0.789 g/mL
-
Calculate:
V ≈ 58.3 mL
Therefore, 1 mole of ethanol occupies approximately 58.3 milliliters.
Using Molar Volume:
The molar volume is the volume occupied by one mole of a substance. For liquids and solids, this value is often provided in data tables. If you know the molar volume (Vm), then the conversion is straightforward:
V = n * Vm
Where:
- V = volume
- n = number of moles
- Vm = molar volume
Practical Applications and Considerations
The ability to convert moles to volume is crucial in numerous chemical contexts:
- Stoichiometry: Many stoichiometric calculations require converting moles of reactants or products to volumes, particularly when dealing with gases involved in reactions.
- Titrations: In titrations, the volume of a solution needed to reach the equivalence point is directly related to the number of moles of the analyte.
- Gas Chromatography: Gas chromatography uses the volume of a gas to quantify the amount of a substance.
- Solution Preparation: Preparing solutions of a specific concentration often involves calculating the volume of solvent needed based on the desired number of moles of solute.
- Environmental Science: Calculating pollutant concentrations in air or water frequently requires converting between moles and volume.
Beyond the Basics: Advanced Considerations
While the methods outlined above are sufficient for many situations, several factors can influence accuracy:
- Temperature and Pressure Effects: For gases, variations in temperature and pressure significantly impact volume. Ensure you use the correct values for these parameters in your calculations.
- Non-Ideal Behavior: Real gases deviate from ideal behavior under certain conditions. For high-pressure or low-temperature situations, employing more complex equations of state is necessary for greater accuracy.
- Substance Purity: The purity of the substance significantly influences its molar mass and density. Impurities can lead to errors in the volume calculations.
- Significant Figures: Always consider the significant figures in your measurements when calculating the final volume to ensure accuracy and avoid misleading precision.
Conclusion
Converting moles to volume is a vital skill in chemistry. Mastering this conversion requires understanding the relationship between moles and volume for different states of matter, utilizing the ideal gas law for gases, and employing density or molar volume for liquids and solids. By carefully considering the specific conditions and applying the appropriate formulas, you can confidently perform these conversions with accuracy and apply them to a wide range of chemical problems and real-world applications. Remember to always account for factors that may affect accuracy, such as non-ideal gas behavior, temperature and pressure fluctuations, and the purity of the substance. With practice and attention to detail, these conversions will become second nature.
Latest Posts
Latest Posts
-
What Are The Membrane Bound Organelles
Mar 19, 2025
-
What Does A Penetration Pricing Demand Curve Look Like
Mar 19, 2025
-
How To Calculate Heat Of Reaction In Kj Mol
Mar 19, 2025
-
What Is Zone Of Inhibition In Microbiology
Mar 19, 2025
-
How To Add Formal Charges To Resonance Structures
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Do You Convert Moles To Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
