Are Aldehydes Or Ketones More Reactive
Muz Play
Mar 25, 2025 · 5 min read
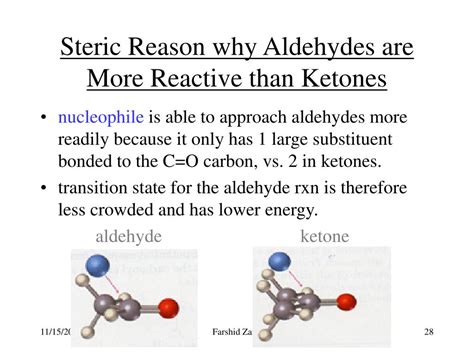
Table of Contents
- Are Aldehydes Or Ketones More Reactive
- Table of Contents
- Are Aldehydes or Ketones More Reactive? A Comprehensive Comparison
- Understanding the Carbonyl Group: The Source of Reactivity
- Structural Differences: The Key to Reactivity Variations
- Steric Hindrance: A Bulky Obstacle
- Electronic Effects: The Influence of Substituents
- Comparing Reactivity in Specific Reactions
- Nucleophilic Addition Reactions
- Oxidation Reactions
- Reduction Reactions
- Factors Affecting Reactivity: A Deeper Dive
- Conclusion: Aldehydes Reign Supreme in Reactivity
- Latest Posts
- Latest Posts
- Related Post
Are Aldehydes or Ketones More Reactive? A Comprehensive Comparison
The question of whether aldehydes or ketones are more reactive is a fundamental one in organic chemistry. While both are carbonyl compounds, sharing the characteristic C=O group, their reactivity differs significantly due to structural variations. This difference stems primarily from the steric hindrance around the carbonyl carbon and the electronic effects of the substituents. This article delves into a comprehensive comparison, exploring the nuances of their reactivity in various reactions and providing a clear understanding of why aldehydes generally exhibit greater reactivity.
Understanding the Carbonyl Group: The Source of Reactivity
Both aldehydes and ketones possess a carbonyl group (C=O), a polar functional group characterized by a significant difference in electronegativity between carbon and oxygen. Oxygen, being more electronegative, pulls electron density away from the carbon atom, creating a partially positive charge (δ+) on the carbon and a partially negative charge (δ-) on the oxygen. This polarization is crucial for the reactivity of both aldehydes and ketones. Nucleophiles, electron-rich species, are attracted to the electrophilic (δ+) carbonyl carbon, while electrophiles target the nucleophilic (δ-) oxygen.
Structural Differences: The Key to Reactivity Variations
The key difference lies in the substituents attached to the carbonyl carbon. Aldehydes have one alkyl or aryl group and one hydrogen atom attached to the carbonyl carbon, while ketones have two alkyl or aryl groups. This seemingly minor difference dramatically impacts steric hindrance and electronic effects, significantly influencing their reactivity.
Steric Hindrance: A Bulky Obstacle
Ketones, with two bulky alkyl or aryl groups surrounding the carbonyl carbon, experience greater steric hindrance than aldehydes. This steric crowding makes it more difficult for nucleophiles to approach and attack the carbonyl carbon. The larger the substituents, the more pronounced this effect becomes. This steric hindrance is a major factor contributing to the lower reactivity of ketones compared to aldehydes. In essence, the bulky groups act as physical barriers, hindering the approach of nucleophiles.
Electronic Effects: The Influence of Substituents
The electronic nature of the substituents also plays a role. Alkyl groups are electron-donating groups (+I effect), which slightly increase the electron density on the carbonyl carbon, making it less electrophilic. Ketones, possessing two alkyl groups, experience a greater +I effect compared to aldehydes, which only have one. This increased electron density further reduces the electrophilicity of the carbonyl carbon in ketones, making them less susceptible to nucleophilic attack.
Comparing Reactivity in Specific Reactions
The differences in reactivity between aldehydes and ketones are vividly illustrated when we examine their behavior in common organic reactions. Let's explore a few key examples:
Nucleophilic Addition Reactions
Nucleophilic addition is a cornerstone reaction for both aldehydes and ketones. This reaction involves the attack of a nucleophile on the electrophilic carbonyl carbon, followed by protonation. Aldehydes generally undergo nucleophilic addition reactions more readily than ketones due to the lower steric hindrance and reduced +I effect mentioned earlier.
Examples of Nucleophilic Addition:
-
Grignard Reaction: Grignard reagents (organomagnesium halides) readily add to both aldehydes and ketones, forming alcohols. However, aldehydes react faster and with higher yields than ketones, especially in the case of sterically hindered ketones.
-
Cyanohydrin Formation: Cyanide ions (CN-) add to the carbonyl group, forming cyanohydrins. Aldehydes react more readily than ketones in this reaction as well.
-
Hydration: The addition of water to the carbonyl group forms gem-diols (hydrates). Aldehydes are generally more readily hydrated than ketones.
Oxidation Reactions
Aldehydes are significantly more easily oxidized than ketones. This is because the oxidation of aldehydes involves the cleavage of the C-H bond adjacent to the carbonyl group, forming a carboxylic acid. Ketones lack this easily oxidized α-hydrogen, making them resistant to oxidation under mild conditions.
Tollens' Test and Fehling's Test: Distinguishing Aldehydes from Ketones
Tollens' test and Fehling's test are classic qualitative tests used to distinguish between aldehydes and ketones. These tests rely on the ability of aldehydes to be oxidized by mild oxidizing agents, while ketones remain unaffected. The positive results are indicated by the formation of a silver mirror in Tollens' test and a red precipitate of cuprous oxide in Fehling's test, both indicative of aldehyde oxidation.
Reduction Reactions
Both aldehydes and ketones can undergo reduction reactions, typically using reducing agents like lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4). These reducing agents add hydride ions (H-) to the carbonyl carbon, converting aldehydes to primary alcohols and ketones to secondary alcohols. While both react, the reaction rates can vary depending on the steric hindrance around the carbonyl group, with less hindered aldehydes generally reacting slightly faster.
Factors Affecting Reactivity: A Deeper Dive
Several factors beyond steric hindrance and electronic effects influence the relative reactivity of aldehydes and ketones:
-
Solvent Effects: The choice of solvent can significantly impact reaction rates. Polar protic solvents often stabilize the transition state in nucleophilic addition reactions, enhancing reactivity.
-
Catalyst Effects: The presence of a catalyst, such as an acid or base, can significantly accelerate the reaction rate by influencing the mechanism of the reaction.
-
Temperature: Higher temperatures generally increase the reaction rate, particularly in reactions with higher activation energies.
-
Nature of the Nucleophile: The strength and nucleophilicity of the attacking species play a crucial role in determining reaction rates. Stronger nucleophiles generally react faster.
Conclusion: Aldehydes Reign Supreme in Reactivity
In summary, while both aldehydes and ketones possess a carbonyl group and undergo similar types of reactions, aldehydes are generally more reactive than ketones. This difference in reactivity is primarily attributed to the lower steric hindrance around the carbonyl carbon in aldehydes and the reduced +I effect from only one alkyl or aryl substituent. This leads to a more electrophilic carbonyl carbon in aldehydes, making them more susceptible to nucleophilic attack and oxidation. The specific reaction conditions, including solvent, catalyst, temperature, and the nature of the nucleophile, also play important roles in determining the precise reactivity of both aldehydes and ketones in any given reaction. Understanding these nuances provides a robust foundation for predicting and manipulating the outcome of organic reactions involving these vital carbonyl compounds. The differences highlighted here are critical in synthetic organic chemistry, allowing chemists to selectively control which carbonyl compound reacts under specific conditions, paving the way for the synthesis of diverse and complex organic molecules.
Latest Posts
Latest Posts
-
Identify The Plant Tissues In The Three Images
Mar 28, 2025
-
When Was The Story Of An Hour Written
Mar 28, 2025
-
Molecular Geometry Vs Electron Pair Geometry
Mar 28, 2025
-
Branching Network Of Intersecting Nerves And Associated Blood Vessels
Mar 28, 2025
-
What Is A Zone Of Inhibition
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Are Aldehydes Or Ketones More Reactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
