Are All Atoms In An Element Identical
Muz Play
Mar 16, 2025 · 6 min read
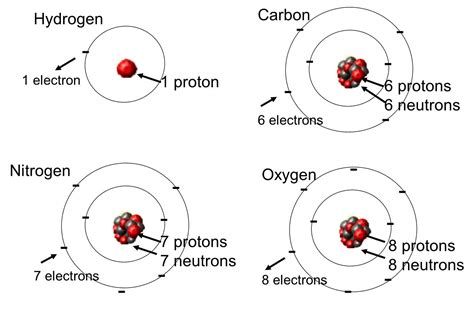
Table of Contents
Are All Atoms in an Element Identical? Delving into Isotopes and Atomic Structure
The simple answer is: no, not all atoms in an element are identical. While the concept of an element suggests a uniform composition, the reality at the atomic level is more nuanced. This article will delve into the fascinating world of atomic structure, isotopes, and the subtle variations that exist within a single element. Understanding these variations is crucial in various scientific fields, including chemistry, physics, and nuclear medicine.
Understanding the Basics: Elements and Atoms
Before exploring the nuances, let's establish a firm understanding of fundamental concepts. An element is a pure substance consisting entirely of one type of atom. These atoms are characterized by their atomic number, which represents the number of protons in the atom's nucleus. The number of protons uniquely defines an element. For example, all atoms with one proton are hydrogen, all atoms with two protons are helium, and so on.
An atom, the fundamental building block of matter, comprises three subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus.
- Neutrons: Neutrally charged particles also found within the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells.
The number of protons dictates the element's identity, while the number of neutrons can vary. This variation leads to the existence of isotopes.
Isotopes: The Key to Atomic Variation
Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons. This difference in neutron count alters the atom's mass number, which is the sum of protons and neutrons. While isotopes of an element share the same chemical properties due to their identical number of protons and electrons, their physical properties, particularly mass, can differ significantly.
For example, consider carbon (atomic number 6). The most common isotope is carbon-12 (¹²C), with six protons and six neutrons. However, carbon also has isotopes such as carbon-13 (¹³C) with six protons and seven neutrons, and carbon-14 (¹⁴C) with six protons and eight neutrons. All three are carbon because they possess six protons, but their differing neutron numbers result in variations in their mass and stability.
Impact of Isotope Variations
The presence of different isotopes significantly influences an element's properties and behavior:
-
Mass Spectrometry: Isotopic variations are routinely measured using mass spectrometry, a technique that separates ions based on their mass-to-charge ratio. This method is crucial in various applications, from determining the age of artifacts (carbon dating using ¹⁴C) to identifying unknown compounds.
-
Nuclear Stability: The neutron-to-proton ratio in an atom's nucleus influences its stability. Certain isotopes are radioactive, meaning their nuclei are unstable and undergo decay, emitting radiation. This radioactive decay is exploited in nuclear medicine for diagnostic and therapeutic purposes. For instance, iodine-131 is used in treating thyroid cancer.
-
Nuclear Reactions: The differences in isotopic masses affect the energy changes during nuclear reactions, such as nuclear fission and fusion. These reactions are central to nuclear power generation and nuclear weapons.
-
Chemical Properties: Although isotopes of an element share the same number of electrons, their slightly different masses can subtly influence reaction rates in some cases. This isotope effect is often minimal but can be crucial in certain high-precision experiments.
Exploring Specific Isotope Examples
Let's delve deeper into specific examples to further illustrate the concept of isotopic variation:
Hydrogen Isotopes: A Striking Example
Hydrogen presents a clear example of significant isotopic variation. It has three isotopes:
- Protium (¹H): One proton and no neutrons. This is the most common isotope.
- Deuterium (²H or D): One proton and one neutron. It's heavier than protium and is used in nuclear fusion research.
- Tritium (³H or T): One proton and two neutrons. It's radioactive and used in certain scientific applications.
The differences in mass between these isotopes are considerable, impacting their physical properties, and even leading to subtle differences in chemical reaction rates. Deuterium oxide (heavy water) for example, exhibits significantly different properties compared to regular water.
Uranium Isotopes: Significance in Nuclear Energy
Uranium, with its two primary isotopes, uranium-235 (²³⁵U) and uranium-238 (²³⁸U), plays a pivotal role in nuclear energy. ²³⁵U is fissile, meaning it can sustain a chain reaction of nuclear fission, releasing vast amounts of energy. ²³⁸U, on the other hand, is not fissile but can be converted to plutonium, another fissile material. The separation of these isotopes is a critical process in nuclear fuel enrichment.
Carbon Isotopes and Carbon Dating
As mentioned earlier, carbon-14 (¹⁴C) is a radioactive isotope used in radiocarbon dating. This technique determines the age of organic materials by measuring the remaining ¹⁴C, which decays at a known rate. The abundance of ¹⁴C in the atmosphere is constant during an organism's lifetime, but after death, it begins to decay, allowing scientists to estimate the time elapsed since the organism died.
Beyond Isotopes: Other Atomic Variations
While isotopes represent the primary variation within an element, other subtle differences exist:
-
Nuclear isomers: These are atoms with the same number of protons and neutrons but different energy states within the nucleus. This difference can affect the atom's stability and decay characteristics.
-
Ionic states: Atoms can gain or lose electrons to form ions, which carry a net electric charge. This alters the atom's chemical properties and behavior. For example, a sodium atom (Na) can lose one electron to become a sodium ion (Na⁺), a positively charged ion.
The Implications of Isotopic Variations
The existence of isotopes has significant implications across various fields:
-
Geochemistry: Isotopic ratios are used to study geological processes, including dating rocks and tracing the movement of elements through the Earth's systems.
-
Environmental science: Isotope analysis helps track pollution sources and understand environmental processes.
-
Medicine: Radioactive isotopes are vital in medical imaging and cancer therapy.
-
Forensic science: Isotopic analysis aids in crime investigations, such as tracing the origin of materials.
Conclusion: Embracing the Nuances of Atomic Structure
In conclusion, while the concept of an element might initially suggest uniformity at the atomic level, the reality is richer and more complex. The existence of isotopes, with their varying neutron numbers, reveals that not all atoms within an element are identical. This subtle variation significantly influences the element's properties and has profound implications across diverse scientific disciplines. Understanding these nuances is crucial for advancing our knowledge of matter and its behavior in various contexts. The variations in isotopes, their impact on nuclear stability, and their applications in diverse fields highlight the intricate and fascinating nature of atomic structure. From carbon dating to nuclear energy, isotopes reveal the power and complexity of the world at its most fundamental level. Further exploration into this realm continues to uncover new insights and expands the boundaries of scientific understanding.
Latest Posts
Latest Posts
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Are All Atoms In An Element Identical . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
