Are Atoms Of A Given Element Identical
Muz Play
Mar 16, 2025 · 6 min read
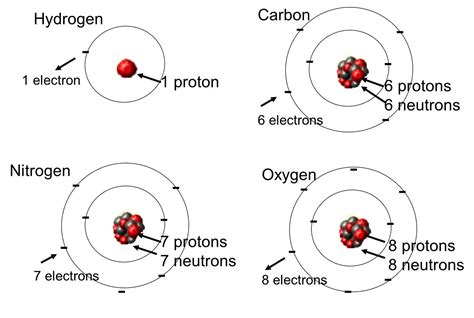
Table of Contents
Are Atoms of a Given Element Identical? Delving into Isotopes and Atomic Structure
The seemingly simple question, "Are atoms of a given element identical?" leads us down a fascinating rabbit hole into the heart of atomic structure and the nuances of elemental identity. The short answer is: no, atoms of a given element are not all identical. While they share defining characteristics, subtle variations exist that profoundly impact their properties and behavior. This exploration will delve into the complexities of isotopes, their impact on atomic mass, and the implications for various scientific fields.
Understanding the Fundamentals: What Defines an Element?
Before we address the nuances of atomic identity, let's establish a firm foundation. An element is defined by its atomic number, which represents the number of protons in its nucleus. This number is unique to each element and dictates its place on the periodic table. For instance, all atoms with one proton are hydrogen, all atoms with two protons are helium, and so on. This proton number dictates the element's chemical properties, determining how it will interact with other elements to form compounds.
The Role of Electrons and the Electron Shell
While the number of protons defines an element, the number of electrons determines its reactivity and chemical behavior. In a neutral atom, the number of electrons equals the number of protons. Electrons occupy energy levels or shells surrounding the nucleus. The outermost shell, known as the valence shell, contains electrons that participate in chemical bonding. The arrangement of these valence electrons is crucial for determining an element's chemical properties and how it will form bonds with other atoms.
The Nucleus: Protons and Neutrons
The nucleus, the atom's core, houses not only protons but also neutrons. Unlike protons, which carry a positive charge, neutrons are electrically neutral. The combined number of protons and neutrons is called the mass number.
Isotopes: The Source of Atomic Variation
Now, we arrive at the crux of the matter: isotopes. Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons. This difference in neutron count leads to variations in the atom's mass but not its chemical properties.
Examples of Isotopes: Carbon-12 and Carbon-14
Consider carbon (atomic number 6). The most common isotope is carbon-12, which has 6 protons and 6 neutrons. However, carbon-14 also exists, possessing 6 protons and 8 neutrons. Both are carbon atoms because they share the same number of protons, but they differ in their mass number (12 and 14, respectively). This difference in neutron number affects the atom's stability and consequently its behavior.
The Impact of Isotopes on Atomic Mass
The atomic mass listed on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element. This weighted average accounts for the relative abundance of each isotope. For example, the atomic mass of carbon is approximately 12.011 amu (atomic mass units), slightly higher than 12, reflecting the presence of heavier isotopes like carbon-13 and carbon-14, albeit in smaller quantities than carbon-12.
Isotopic Abundance and Its Significance
The abundance of each isotope varies naturally. Some isotopes are more stable than others. For instance, carbon-12 is the most abundant carbon isotope, while carbon-14 is radioactive and decays over time. This radioactive decay makes carbon-14 incredibly valuable for radiocarbon dating, a technique used to determine the age of organic materials.
Stable vs. Radioactive Isotopes
Stable isotopes do not undergo radioactive decay. They remain unchanged over time. Radioactive isotopes, on the other hand, are unstable and undergo spontaneous decay, emitting particles or energy to reach a more stable state. The rate of decay is constant and characteristic of each radioactive isotope. This property is used in various applications, including medical imaging and cancer therapy.
Implications Across Scientific Disciplines
The existence of isotopes has significant implications across numerous scientific disciplines:
1. Chemistry: Isotopic Effects
Isotopes, despite their identical chemical behavior, can exhibit slight differences in their reaction rates and equilibrium constants, known as isotopic effects. These effects are generally small but can be measurable and significant in certain reactions, particularly those involving lighter elements. Understanding isotopic effects is crucial for accurate chemical modeling and analysis.
2. Physics: Nuclear Reactions and Applications
In nuclear physics, isotopes are central to understanding nuclear reactions, nuclear fission, and nuclear fusion. The stability and decay properties of specific isotopes are vital in the development of nuclear power and nuclear weapons. Furthermore, isotopic separation techniques are crucial for various applications, including medical isotopes used in diagnostics and therapies.
3. Geology and Archaeology: Isotopic Dating
Radioactive isotopes are essential tools in geology and archaeology for dating rocks, fossils, and other materials. Techniques like radiocarbon dating (using carbon-14) and uranium-lead dating allow scientists to determine the age of samples and gain insights into Earth's history and the evolution of life.
4. Biology and Medicine: Tracer Studies and Medical Applications
Stable and radioactive isotopes find widespread applications in biological and medical research. Stable isotopes are used as tracers in metabolic studies, allowing scientists to follow the pathways of molecules within organisms. Radioactive isotopes are used in medical imaging techniques like PET (positron emission tomography) scans, providing crucial information for diagnosis and treatment planning. They also find therapeutic applications in targeted radiation therapy.
5. Environmental Science: Isotopic Tracers and Monitoring
Isotopes serve as powerful tracers in environmental science. By analyzing the isotopic composition of water, air, and soil samples, scientists can track pollution sources, monitor the movement of pollutants, and investigate various environmental processes. For instance, isotopic analysis can help identify the origin of groundwater or track the movement of contaminants in rivers and oceans.
Conclusion: A Nuance of Identity
In conclusion, the seemingly simple question of whether atoms of a given element are identical reveals a complex and fascinating truth. While all atoms of an element share the same number of protons and thus the same chemical identity, the variations in neutron number (isotopes) lead to differences in mass and potentially subtle differences in behavior. The study of isotopes is crucial across numerous scientific disciplines, contributing to our understanding of fundamental processes in chemistry, physics, geology, biology, medicine, and environmental science. The existence of isotopes highlights the richness and complexity of the atomic world and underscores the importance of considering these variations for a comprehensive understanding of the natural world. The seemingly simple atom is, in fact, far more complex and nuanced than one might initially assume. This multifaceted nature is what makes the study of atomic structure both challenging and deeply rewarding.
Latest Posts
Latest Posts
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
-
Evidence Of Light As A Particle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Are Atoms Of A Given Element Identical . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
