Are D Sugars More Abundant In Nature Than L-sugars
Muz Play
Mar 26, 2025 · 5 min read
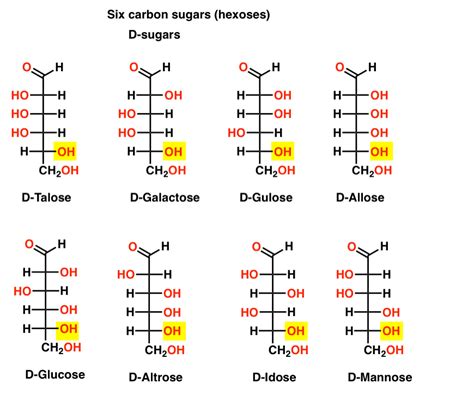
Table of Contents
Are D-Sugars More Abundant in Nature Than L-Sugars? A Deep Dive into the Chirality of Carbohydrates
The world of carbohydrates is a fascinating one, filled with intricate structures and crucial biological roles. Central to understanding these roles is the concept of chirality – the existence of molecules as mirror images, known as enantiomers. In the case of sugars, this manifests as D-sugars and L-sugars. But which form dominates in nature? The simple answer is D-sugars are overwhelmingly more abundant than L-sugars in nature. This article will delve into the reasons behind this dominance, exploring the chemical properties, biological pathways, and evolutionary implications of this striking asymmetry.
Understanding D- and L-Sugars: A Matter of Chirality
Before diving into the abundance question, let's establish a clear understanding of D- and L-sugars. These designations refer to the stereochemistry of the molecule, specifically the configuration around the chiral carbon furthest from the carbonyl group (aldehyde or ketone). This carbon is often referred to as the penultimate carbon.
-
D-sugars: In D-sugars, the hydroxyl group (-OH) on this penultimate carbon is positioned on the right side when the molecule is represented in Fischer projection.
-
L-sugars: Conversely, in L-sugars, the hydroxyl group on the penultimate carbon is positioned on the left side in the Fischer projection.
It's crucial to remember that D- and L- designations are not related to the optical rotation of the sugar (dextrorotatory or levorotatory). While many D-sugars are dextrorotatory (+), and many L-sugars are levorotatory (-), this is not always the case. The D/L notation solely reflects the absolute configuration at the penultimate chiral center.
The Overwhelming Prevalence of D-Sugars: Why the Asymmetry?
The remarkable dominance of D-sugars in biological systems remains a topic of ongoing scientific inquiry. There's no single definitive answer, but several hypotheses attempt to explain this phenomenon:
1. The Primordial Soup and Early Life's Choices
One compelling theory suggests that the initial selection of D-sugars might have been a matter of chance in the prebiotic "primordial soup." Perhaps a slight initial excess of D-sugars, due to random processes or even extraterrestrial influences, provided a selective advantage to early life forms that utilized these sugars. Once metabolic pathways evolved to efficiently process D-sugars, a positive feedback loop was established, making a switch to L-sugars exceedingly difficult. This early selection event effectively set the stage for the dominance of D-sugars we observe today.
2. Enzymatic Specificity and Metabolic Pathways
Enzymes, the biological catalysts that drive metabolic reactions, exhibit remarkable specificity. They are typically designed to interact with only one enantiomer, either D or L. The evolution of life has resulted in enzymes that primarily interact with D-sugars. This is a crucial factor driving the abundance of D-sugars. Consider the enzymes involved in glycolysis, the central metabolic pathway for glucose breakdown. These enzymes are specifically designed for D-glucose, effectively excluding L-glucose from the process.
3. Kinetic and Thermodynamic Advantages
Some research suggests that D-sugars might possess subtle kinetic or thermodynamic advantages over their L-counterparts in certain biochemical reactions. These advantages, though possibly minor individually, could have cumulatively contributed to the selection pressure favoring D-sugars over evolutionary time. The exact nature of these advantages, however, remains an active area of research.
4. Self-Assembly and Molecular Recognition
The chirality of sugars influences how they self-assemble and interact with other molecules. The specific three-dimensional structure of D-sugars may have facilitated the formation of more stable and functional biomolecules in early life. This could have further reinforced the selection for D-sugars.
Exceptions to the Rule: The Occurrence of L-Sugars
While D-sugars overwhelmingly dominate, it's essential to acknowledge that L-sugars do exist in nature, albeit in far smaller quantities. Some examples include:
-
L-arabinose: Found in various plant polysaccharides and bacterial cell walls.
-
L-fucose: A component of certain glycoproteins and glycolipids.
-
L-rhamnose: Present in some plant glycosides and bacterial polysaccharides.
The presence of L-sugars in these specific contexts highlights the adaptability of biological systems and demonstrates that the complete exclusion of L-sugars was never absolute. Their presence often reflects specialized functional roles where their specific stereochemistry provides an advantage.
The Significance of Sugar Chirality in Biology and Medicine
The chirality of sugars has profound implications across various biological and medical fields:
-
Drug Design: Understanding the stereochemistry of sugars is crucial in pharmaceutical drug design. Many drugs interact with carbohydrate-binding proteins, and the stereochemical configuration of the sugar component can significantly influence the drug's effectiveness, toxicity, and overall pharmacological profile.
-
Immunology: The immune system recognizes and responds to specific carbohydrate structures on the surfaces of cells and pathogens. The chirality of these sugars plays a key role in this recognition process.
-
Glycobiology: The study of glycosylation, the process of attaching sugars to proteins and lipids, is a rapidly expanding field with implications for understanding various diseases, including cancer and infectious diseases. The chirality of sugars is essential to understanding the diversity and functionality of glycans.
-
Food Science and Nutrition: The chirality of sugars affects their digestibility, metabolism, and overall nutritional value. Understanding these aspects is important for developing foods with improved nutritional profiles.
Conclusion: A Story of Evolutionary Selection
The prevalence of D-sugars in nature is a compelling example of evolutionary selection. While the exact reasons for this dominance remain a subject of ongoing research, it's clear that a combination of factors – including early chance events, enzymatic specificity, and potential kinetic/thermodynamic advantages – likely contributed to the establishment of D-sugars as the dominant form in biological systems. While L-sugars play minor, yet vital, roles, the remarkable asymmetry in the distribution of D- and L-sugars underscores the profound influence of chirality on the evolution and function of life. Further research continues to unravel the intricacies of this fascinating aspect of carbohydrate chemistry and biology. Understanding this asymmetry provides valuable insights into the fundamental principles of life and opens avenues for advancements in various fields, from drug discovery to disease treatment. The story of D-sugar dominance is not merely a chemical curiosity; it's a testament to the power of evolutionary forces shaping the world around us.
Latest Posts
Latest Posts
-
What Is The Distance Between And On The Number Line
Mar 29, 2025
-
Limit Of A Function Of Two Variables
Mar 29, 2025
-
The Short Run Phillips Curve Implies There Is A Trade Off Between
Mar 29, 2025
-
5 Conditions For Hardy Weinberg Equilibrium
Mar 29, 2025
-
Writing The Lewis Structures For A Molecule With Resonance
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Are D Sugars More Abundant In Nature Than L-sugars . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
