Writing The Lewis Structures For A Molecule With Resonance
Muz Play
Mar 29, 2025 · 6 min read
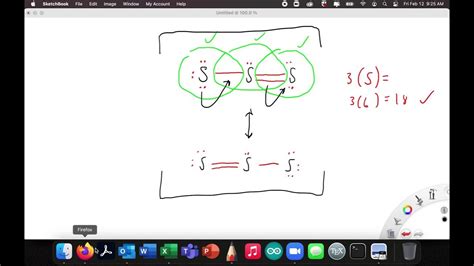
Table of Contents
Writing Lewis Structures for Molecules with Resonance: A Comprehensive Guide
Resonance structures are a crucial concept in chemistry used to depict molecules whose bonding cannot be adequately represented by a single Lewis structure. This article provides a comprehensive guide to drawing Lewis structures, focusing specifically on molecules exhibiting resonance. We'll explore the rules, the implications, and the importance of understanding resonance for accurately representing molecular behavior.
Understanding Lewis Structures and Their Limitations
Before delving into resonance, let's review the basics of Lewis structures. These diagrams use dots to represent valence electrons and lines to represent covalent bonds (shared electron pairs). The goal is to achieve a stable octet (or duet for hydrogen) for each atom in the molecule.
Steps to Drawing a Lewis Structure:
-
Count valence electrons: Add up the valence electrons from all atoms in the molecule. Remember to account for the charge if the molecule is an ion.
-
Identify the central atom: This is usually the least electronegative atom (excluding hydrogen, which is always terminal).
-
Connect atoms with single bonds: Place a single bond (one line) between the central atom and each surrounding atom.
-
Distribute remaining electrons: Place the remaining electrons as lone pairs around the atoms to satisfy the octet rule. Start with the outer atoms, then fill the octet of the central atom.
-
Check for octet rule satisfaction: Ensure all atoms (except hydrogen) have eight valence electrons around them.
Example: CO₂
-
Valence electrons: C (4) + O (6) x 2 = 16
-
Central atom: Carbon
-
Single bonds: O-C-O
-
Remaining electrons: 16 - 4 = 12. Distribute these as lone pairs around the oxygen atoms.
-
Octet check: Each oxygen has 8 electrons, but carbon only has 4. This is where we need double bonds. To give carbon an octet, we move two lone pairs from each oxygen to form double bonds with carbon.
Introducing Resonance Structures
The problem arises when a single Lewis structure cannot accurately represent the bonding in a molecule. This often happens when multiple valid arrangements of electrons can be drawn, all contributing to the overall structure. These multiple structures are called resonance structures, and the actual molecule is a hybrid of these structures – a blend of all contributing forms. It's crucial to understand that the molecule doesn't flip between these structures; it exists as a single, stable entity represented by the average of the contributing resonance structures.
Characteristics of Molecules with Resonance:
- Delocalized electrons: Electrons are not localized to a single bond or atom but are spread across multiple atoms or bonds.
- Multiple valid Lewis structures: Several equally plausible Lewis structures can be drawn, differing only in the placement of electrons.
- Lower overall energy: The resonance hybrid is generally more stable and has lower energy than any individual resonance structure.
- Equal bond lengths: In cases like benzene, resonance leads to equal bond lengths between atoms, even though some resonance structures show single and double bonds.
Drawing Resonance Structures: A Step-by-Step Approach
Let's illustrate with a classic example: the nitrate ion (NO₃⁻).
-
Count valence electrons: N (5) + O (6) x 3 + 1 (negative charge) = 24
-
Central atom: Nitrogen
-
Single bonds: Connect the nitrogen to each oxygen with single bonds.
-
Distribute remaining electrons: 24 - 6 = 18. Place lone pairs around the oxygens to satisfy their octets. Nitrogen will have only 6 electrons.
-
Form double bonds: To give nitrogen an octet, we can move a lone pair from one of the oxygens to form a double bond with nitrogen. However, we can do this with any of the three oxygen atoms. This leads to three equivalent resonance structures.
Resonance Structures of Nitrate Ion (NO₃⁻):
O- O
|| |
-O-N-O -O-N-O O-N-O-
| || ||
O- O O
Notice how the only difference between the structures is the placement of the double bond. The actual nitrate ion is a hybrid of these three structures, with the negative charge delocalized across all three oxygen atoms. The bond order between nitrogen and each oxygen is 1.33 (a blend of one single and two double bonds).
Recognizing Molecules with Resonance
Many molecules exhibit resonance. Some common examples include:
- Carbonate ion (CO₃²⁻): Similar to nitrate, the negative charge is delocalized across the oxygen atoms.
- Benzene (C₆H₆): The six carbon atoms form a ring with alternating single and double bonds. In reality, the bonds are all equal in length, due to resonance.
- Ozone (O₃): The central oxygen atom has a double bond to one oxygen and a single bond to the other. The structure actually has two resonance forms.
- Acetate ion (CH₃COO⁻): The negative charge is delocalized between the two oxygen atoms.
- Sulphate ion (SO₄²⁻): The negative charges are delocalized among the oxygen atoms.
- Carbonate ion (CO₃²⁻): The negative charge is distributed over the three oxygen atoms through resonance.
Formal Charges and Resonance Structures
Formal charges are useful for evaluating the plausibility of resonance structures. A formal charge is the difference between the number of valence electrons an atom has in its neutral state and the number of electrons it "owns" in the Lewis structure. A lower sum of formal charges generally indicates a more stable resonance structure.
Calculating formal charge:
Formal charge = (Valence electrons) - (Non-bonding electrons) - (1/2 x Bonding electrons)
Lower formal charges and structures where negative charges reside on more electronegative atoms are usually more stable.
Resonance Hybrids and the True Molecular Structure
It’s critical to remember that resonance structures are not isomers; they are simply different ways of representing the same molecule. The true structure of the molecule is a resonance hybrid, an average of all the contributing resonance structures. This hybrid is often represented by drawing the molecule with dashed lines for delocalized bonds or by showing the average bond order.
Importance of Understanding Resonance
Understanding resonance is essential for accurately predicting:
- Molecular geometry: The delocalization of electrons influences the shape and bond angles of molecules.
- Reactivity: Resonance stabilization affects how a molecule reacts with other substances.
- Spectroscopic properties: Resonance affects the absorption and emission of light by molecules, which is crucial in spectroscopic analysis.
- Stability: Resonance significantly increases the stability of molecules, making them less reactive.
Advanced Concepts in Resonance
For a more in-depth understanding, consider exploring these advanced concepts:
- Resonance energy: The difference in energy between the actual molecule and the most stable resonance structure.
- Qualitative vs. quantitative approaches to resonance: While Lewis structures provide a qualitative understanding, advanced computational methods can provide quantitative insights into resonance stabilization.
- Aromaticity: A special type of resonance stabilization observed in cyclic, planar molecules with delocalized pi electrons.
Conclusion
Drawing Lewis structures for molecules with resonance is a fundamental skill in chemistry. By understanding the rules of Lewis structures, recognizing the characteristics of molecules exhibiting resonance, and mastering the process of drawing and evaluating resonance structures, you gain valuable insight into molecular properties and reactivity. Remember, the resonance hybrid, not any single resonance structure, represents the true molecule. This comprehensive understanding is vital for success in advanced chemistry courses and for application in various scientific fields. The ability to visualize and interpret resonance structures is a testament to a deeper comprehension of chemical bonding and molecular behavior.
Latest Posts
Latest Posts
-
The Fundamental Unit Of Life Is The
Mar 31, 2025
-
Political Map Of North Africa And Southwest Asia
Mar 31, 2025
-
Work Is The Integral Of Force
Mar 31, 2025
-
Is Water A Reactant Or Product
Mar 31, 2025
-
Four Ways To Represent A Function
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Writing The Lewis Structures For A Molecule With Resonance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
