Are Double Bonds Stronger Than Single Bonds
Muz Play
Mar 27, 2025 · 6 min read
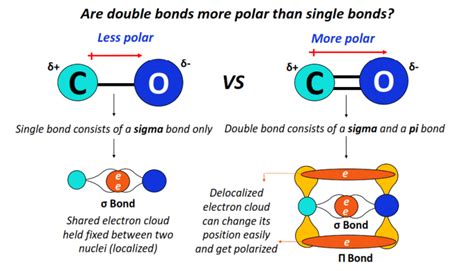
Table of Contents
Are Double Bonds Stronger Than Single Bonds? A Deep Dive into Chemical Bonding
The question of whether double bonds are stronger than single bonds is a fundamental one in chemistry. The short answer is yes, but understanding why requires a deeper exploration of chemical bonding, orbital hybridization, and bond energies. This article will delve into these concepts, providing a comprehensive explanation suitable for both students and anyone curious about the intricacies of molecular structure.
Understanding Chemical Bonds: The Foundation
Before comparing single and double bonds, let's establish a solid understanding of what constitutes a chemical bond. A chemical bond is a lasting attraction between atoms, ions, or molecules that enables the formation of chemical compounds. These bonds arise from the electrostatic forces between oppositely charged particles: electrons and protons. There are several types of chemical bonds, but the most relevant for our discussion are covalent bonds, which are formed by the sharing of electrons between atoms.
Covalent Bonds: Sharing is Caring (Electrons, That Is)
Covalent bonds are formed when atoms share one or more pairs of electrons. The shared electrons are attracted to the nuclei of both atoms, creating a strong attractive force that holds them together. The strength of a covalent bond depends on several factors, including the electronegativity of the atoms involved and the number of electron pairs shared.
Single Bonds: The Baseline
A single bond, also known as a sigma (σ) bond, is formed by the sharing of one pair of electrons between two atoms. This shared pair occupies a region of space directly between the two nuclei, creating a relatively strong bond. Single bonds are generally flexible, allowing for rotation around the bond axis. This rotation contributes to the molecule's flexibility and conformational diversity.
Examples of Single Bonds:
- Ethane (C₂H₆): Each carbon atom in ethane is bonded to three hydrogen atoms and another carbon atom via single bonds.
- Methane (CH₄): The central carbon atom in methane forms four single bonds with four hydrogen atoms.
- Water (H₂O): The oxygen atom in water forms two single bonds with two hydrogen atoms.
Double Bonds: Strength in Numbers (Electron Pairs)
A double bond consists of two pairs of shared electrons between two atoms. It's composed of one sigma (σ) bond and one pi (π) bond. The sigma bond is formed by the direct overlap of atomic orbitals, similar to a single bond. The pi (π) bond, however, is formed by the sideways overlap of p orbitals above and below the sigma bond. This sideways overlap results in a region of electron density above and below the plane of the sigma bond.
The Significance of Pi Bonds:
The pi bond is crucial in understanding why double bonds are stronger than single bonds. While the sigma bond provides the primary attractive force, the pi bond adds an additional layer of electron density, increasing the overall attractive force between the two atoms. This additional attraction translates to a higher bond strength.
Examples of Double Bonds:
- Ethene (C₂H₄): The two carbon atoms in ethene are bonded together by a double bond, consisting of one sigma and one pi bond. Each carbon atom also forms two single bonds with hydrogen atoms.
- Carbon Dioxide (CO₂): Each carbon atom in carbon dioxide is double-bonded to each oxygen atom.
- Oxygen (O₂): The oxygen molecule is held together by a double bond.
Bond Energy: A Quantitative Measure of Bond Strength
Bond energy is the amount of energy required to break one mole of a particular type of bond in the gaseous state. It's a direct measure of bond strength: the higher the bond energy, the stronger the bond. Experimental data consistently shows that double bonds have significantly higher bond energies than single bonds.
Comparing Bond Energies:
Consider the carbon-carbon bond. A single C-C bond has a bond energy of approximately 348 kJ/mol, whereas a double C=C bond has a bond energy of approximately 614 kJ/mol. This difference clearly demonstrates that the double bond is substantially stronger. The same trend holds true for other types of double bonds compared to their single-bond counterparts.
Orbital Hybridization: Shaping the Bonds
The strength of a double bond can also be explained through the concept of orbital hybridization. Hybridization is the mixing of atomic orbitals to form new hybrid orbitals that are more suitable for bonding. In the case of carbon atoms involved in double bonds, sp² hybridization is common.
sp² Hybridization and Double Bonds:
In sp² hybridization, one s orbital and two p orbitals combine to form three sp² hybrid orbitals, which are arranged in a trigonal planar geometry. The remaining p orbital is unhybridized and participates in the formation of the pi (π) bond. This arrangement optimizes the overlap of atomic orbitals, contributing to the increased strength of the double bond.
Bond Length: A Consequence of Bond Strength
Bond length is the average distance between the nuclei of two bonded atoms. Generally, stronger bonds are shorter than weaker bonds. Since double bonds are stronger than single bonds, they also tend to be shorter.
Comparing Bond Lengths:
The C-C single bond has a typical length of around 1.54 Å (angstroms), while the C=C double bond is significantly shorter, at approximately 1.34 Å. This shorter bond length reflects the increased electron density and stronger attractive force between the carbon atoms in the double bond.
Double Bonds and Rigidity: A Note on Rotation
Unlike single bonds, which allow for free rotation around the bond axis, double bonds exhibit restricted rotation. The pi (π) bond prevents rotation because breaking the pi bond requires significant energy. This restricted rotation leads to the existence of isomers, known as cis-trans isomers or geometric isomers. This rigidity is a characteristic feature stemming from the double bond's enhanced strength and structure.
Beyond Double Bonds: Triple Bonds
Taking the concept further, we can also consider triple bonds, which consist of three pairs of shared electrons between two atoms (one sigma and two pi bonds). Triple bonds are even stronger and shorter than double bonds, reflecting the even greater electron density and attractive force between the atoms. Examples of triple bonds include the nitrogen-nitrogen bond in N₂ and the carbon-carbon bond in alkynes.
Real-world implications of double bond strength:
The strength and rigidity imparted by double bonds have significant implications in various fields:
- Polymers: The presence of double bonds in polymers like polyethylene influences their mechanical properties, determining their flexibility or rigidity.
- Biological Molecules: Double bonds in fatty acids influence their melting points and impact the fluidity of cell membranes.
- Industrial Chemistry: The strength of double bonds plays a crucial role in many chemical reactions and industrial processes, including polymer synthesis and catalytic reactions.
- Spectroscopy: The unique electronic structure of double bonds allows for their identification and characterization through various spectroscopic techniques.
Conclusion: Double Bonds – A Stronger Force
In summary, double bonds are indeed stronger than single bonds due to the presence of both sigma and pi bonds, resulting in higher bond energies, shorter bond lengths, and restricted rotation. The enhanced strength of double bonds stems from the increased electron density and stronger attractive forces between the atoms, profoundly influencing molecular structure, reactivity, and physical properties. Understanding this fundamental difference is key to grasping the behavior and properties of countless molecules in various scientific and industrial contexts. From the everyday materials we use to the intricate molecules sustaining life, the strength of the double bond plays a pivotal role.
Latest Posts
Latest Posts
-
What Is A Reference Group In Sociology
Mar 31, 2025
-
Dividing Polynomials Math Lib Answer Key
Mar 31, 2025
-
What Is Held Constant In Gay Lussacs Law
Mar 31, 2025
-
Example Of A Line In A Poem
Mar 31, 2025
-
Interval Of Convergence Of A Taylor Series
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Are Double Bonds Stronger Than Single Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
