Are Fatty Acids Polar Or Nonpolar
Muz Play
Mar 20, 2025 · 5 min read
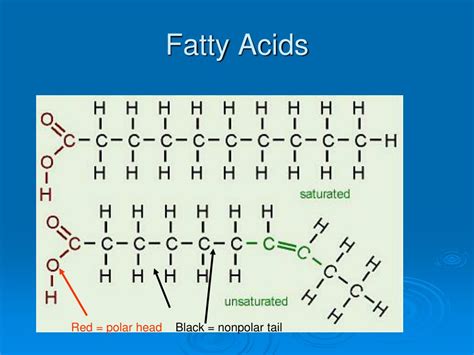
Table of Contents
Are Fatty Acids Polar or Nonpolar? Understanding the Chemistry of Fats
Fatty acids are fundamental components of lipids, playing crucial roles in various biological processes. Understanding their polarity is key to comprehending their behavior and function in living organisms. This detailed article explores the polarity of fatty acids, examining their chemical structure, the influence of saturation and unsaturation, and the implications of their polarity on their properties and biological roles.
The Chemical Structure of Fatty Acids: A Foundation for Polarity
Fatty acids are long hydrocarbon chains with a carboxyl group (-COOH) at one end. This carboxyl group is the key player in determining the overall polarity of the molecule. The hydrocarbon chain itself, comprised of carbon and hydrogen atoms, is largely nonpolar. This is because the electronegativity difference between carbon and hydrogen is minimal, resulting in a relatively even distribution of electrons.
The Polar Carboxyl Head: The Exception to the Rule
The carboxyl group, however, is polar. The oxygen atoms are significantly more electronegative than both carbon and hydrogen. This electronegativity difference creates a polar bond within the carboxyl group, resulting in a partial negative charge (δ-) on the oxygen atoms and a partial positive charge (δ+) on the carbon atom. This uneven electron distribution makes the carboxyl group hydrophilic, meaning it has an affinity for water.
Saturation vs. Unsaturation: A Defining Factor in Polarity
The degree of saturation in a fatty acid significantly impacts its overall polarity. Let's delve into the details:
Saturated Fatty Acids: Mostly Nonpolar
Saturated fatty acids have only single bonds between their carbon atoms. This results in a straight, relatively compact structure. While the carboxyl group remains polar, the long, nonpolar hydrocarbon chain dominates the molecule's overall properties. Consequently, saturated fatty acids are considered largely nonpolar. Their interaction with water is limited, and they tend to aggregate together, forming hydrophobic interactions. This explains why saturated fats tend to be solid at room temperature.
Unsaturated Fatty Acids: Introducing Polarity Through Double Bonds
Unsaturated fatty acids contain one or more double bonds between their carbon atoms. These double bonds introduce kinks or bends in the hydrocarbon chain. The presence of these double bonds subtly alters the electron distribution within the molecule. The double bonds can create localized regions of higher electron density, making these areas slightly more polar than the rest of the chain.
Monounsaturated vs. Polyunsaturated Fatty Acids
-
Monounsaturated fatty acids: Possess one double bond, introducing a single bend in the hydrocarbon chain. Their polarity is still largely dictated by the nonpolar hydrocarbon chain, but the presence of the double bond introduces a small element of increased polarity compared to saturated fatty acids.
-
Polyunsaturated fatty acids: Contain multiple double bonds, resulting in several kinks in the hydrocarbon chain. The cumulative effect of these double bonds makes the overall molecule slightly more polar than monounsaturated fatty acids. The increased polarity results in slightly greater interaction with water.
The Amphipathic Nature of Fatty Acids
The presence of both polar (carboxyl group) and nonpolar (hydrocarbon chain) regions within a fatty acid molecule gives them an amphipathic nature. This means they possess both hydrophilic (water-loving) and hydrophobic (water-fearing) characteristics. This dual nature is crucial to their functions in biological membranes.
Amphipathic Behavior in Biological Membranes
In biological membranes, fatty acids form lipid bilayers. The hydrophilic carboxyl groups of the fatty acids face outwards, interacting with the aqueous environment (cytoplasm and extracellular fluid), while the hydrophobic hydrocarbon chains cluster together in the interior of the bilayer, shielding themselves from water. This arrangement is thermodynamically favorable, ensuring the stability and integrity of the cell membrane.
Implications of Fatty Acid Polarity: Biological Roles and Properties
The polarity of fatty acids profoundly influences their physical and chemical properties, as well as their biological functions.
1. Membrane Fluidity: A Dance Between Polarity and Saturation
The fluidity of cell membranes is significantly impacted by the degree of saturation of the fatty acids within them. Unsaturated fatty acids, with their kinked structures, prevent tight packing of the hydrocarbon chains, leading to increased membrane fluidity. Conversely, saturated fatty acids, with their straight chains, pack more tightly, resulting in less fluid membranes. This fluidity is crucial for various cellular processes such as membrane protein function and nutrient transport.
2. Fatty Acid Interactions: Hydrophobic and Hydrophilic Forces
The polarity of fatty acids determines their interactions with other molecules. The hydrophobic hydrocarbon chains drive interactions through van der Waals forces and hydrophobic interactions. The polar carboxyl group participates in hydrogen bonding and other polar interactions with water and other polar molecules. These interactions are essential for various biological processes, including enzyme activity, protein folding, and signal transduction.
3. Emulsification: Bridging the Gap Between Polar and Nonpolar Worlds
The amphipathic nature of fatty acids enables them to act as emulsifiers. Emulsifiers stabilize mixtures of oil and water by reducing the interfacial tension between the two phases. This is because the hydrophilic carboxyl group interacts with water, while the hydrophobic hydrocarbon chain interacts with oil, creating a stable emulsion. This property is utilized in various industrial applications, such as food processing and cosmetics.
4. Solubility: The Importance of the Polarity Balance
The solubility of fatty acids in water is dictated by their polarity. Shorter-chain fatty acids, with a relatively higher proportion of polar carboxyl group to nonpolar hydrocarbon chain, are more soluble in water than long-chain fatty acids. Longer-chain fatty acids, with their predominantly nonpolar character, are essentially insoluble in water.
5. Melting Points: The Impact of Intermolecular Forces
The melting points of fatty acids are significantly influenced by the degree of saturation and chain length. Saturated fatty acids, which pack more closely due to their straight chains, exhibit higher melting points than unsaturated fatty acids, whose kinked structures hinder efficient packing. Longer-chain fatty acids also tend to have higher melting points than shorter-chain fatty acids due to the increased strength of van der Waals interactions.
Conclusion: A Comprehensive Overview of Fatty Acid Polarity
The polarity of fatty acids, governed primarily by the presence and nature of the carboxyl group and the degree of saturation of the hydrocarbon chain, profoundly influences their behavior and functions in biological systems. Understanding the amphipathic nature of fatty acids and the implications of their polarity on membrane fluidity, interactions with other molecules, emulsification, solubility, and melting points is critical for grasping their diverse roles in biological processes. Further research continues to unravel the intricate interplay between fatty acid polarity and its profound influence on cellular function and overall health. This knowledge has significant implications for various fields, including medicine, nutrition, and biotechnology.
Latest Posts
Latest Posts
-
What Is The Serial Position Curve
Mar 21, 2025
-
Label The Microscopic Anatomy Of Cardiac Muscle
Mar 21, 2025
-
Definition Of A Model In Psychology
Mar 21, 2025
-
Scores Can Be Assumed To Be Symmetric Meaning
Mar 21, 2025
-
How Are Carbohydrates And Lipids Different
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Are Fatty Acids Polar Or Nonpolar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
