Are Metals On The Right Side O The Periodic Table
Muz Play
Mar 24, 2025 · 6 min read
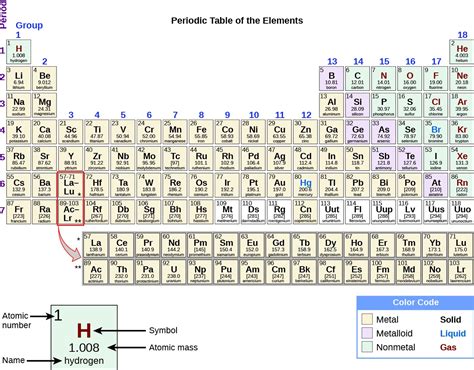
Table of Contents
Are Metals on the Right Side of the Periodic Table? Understanding the Periodic Trends of Metallic Character
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. One crucial property is metallic character, which dictates an element's ability to conduct electricity, form positive ions, and exhibit other characteristic metallic behaviors. A common misconception revolves around the location of metals on the table. The simple answer is no, metals are predominantly found on the left side of the periodic table, not the right. This article delves deeper into the reasons behind this, exploring the periodic trends influencing metallic character and examining the exceptions that add complexity to this seemingly straightforward classification.
Understanding Metallic Character
Metallic character is fundamentally linked to an atom's ability to lose electrons. Elements with low ionization energies – the energy required to remove an electron – readily lose electrons, forming positive ions (cations). This electron loss is crucial to the characteristic properties of metals:
- Electrical Conductivity: The readily available electrons in the outer shell are delocalized, forming a "sea" of electrons that can move freely, enabling efficient electrical conduction.
- Thermal Conductivity: This "sea" of electrons also facilitates the efficient transfer of heat energy.
- Malleability and Ductility: The non-directional nature of metallic bonding allows metal atoms to slide past each other without fracturing the structure, resulting in malleability (ability to be hammered into sheets) and ductility (ability to be drawn into wires).
- Metallic Luster: The interaction of light with the delocalized electrons gives metals their characteristic shine.
Periodic Trends and Metallic Character
The periodic table's arrangement reflects trends in atomic properties. Moving across a period (from left to right), the effective nuclear charge increases. This means the positive charge of the nucleus experienced by the outermost electrons increases as the number of protons grows while the shielding effect of inner electrons remains relatively constant. This increased effective nuclear charge pulls the outermost electrons closer to the nucleus, making them harder to remove. Consequently, metallic character decreases across a period.
Moving down a group (from top to bottom), the atomic radius increases significantly. The outermost electrons are further away from the nucleus, shielded by increasing numbers of inner electrons. This results in a weaker attraction between the nucleus and the valence electrons, making them easier to lose. Hence, metallic character increases down a group.
The Location of Metals on the Periodic Table
Based on these trends, it becomes clear why metals predominantly reside on the left side of the periodic table. Elements on the far left (Groups 1 and 2, the alkali and alkaline earth metals) have very low ionization energies, readily losing one or two electrons to achieve a stable electron configuration. Their high metallic character is evident in their excellent conductivity, malleability, and ductility. Transition metals, located in the center of the table, also exhibit strong metallic character, although their properties are more varied due to the involvement of d-electrons in bonding.
Non-Metals and Metalloids: The Right Side of the Periodic Table
In contrast, elements on the right side of the periodic table (Groups 14-18) have higher ionization energies. These are predominantly non-metals, which tend to gain electrons to achieve a stable electron configuration, forming negative ions (anions). Their properties are very different from metals: they are generally poor conductors of electricity and heat, brittle, and lack metallic luster.
Between the metals and non-metals lies a region of elements known as metalloids (or semi-metals). These elements, including boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po), exhibit properties intermediate between metals and non-metals. They may exhibit some metallic characteristics under certain conditions, but their behavior is often dependent on temperature, pressure, or other factors. Their conductivity, for example, can be semiconductor-like, meaning it increases with increasing temperature, unlike the typical metallic behavior.
Exceptions and Nuances
While the general trend is clear, there are exceptions and nuances that complicate the simple left-vs-right categorization of metals:
- Hydrogen (H): Although placed in Group 1, hydrogen is a non-metal under standard conditions. Its single electron is easily lost in some reactions, but it also readily gains an electron to form the hydride ion (H⁻).
- Transition Metals: While generally metallic, transition metals exhibit a wider range of properties compared to alkali or alkaline earth metals. Some exhibit variable oxidation states, forming ions with different charges, and their bonding characteristics are influenced by the involvement of d-electrons, leading to variations in conductivity and other metallic properties.
- Lanthanides and Actinides: These elements, located at the bottom of the periodic table, are all metals, exhibiting strong metallic character. However, their chemical behavior is often complex due to the influence of f-electrons in their electron configuration.
- Post-Transition Metals: These metals, including elements like aluminum (Al), tin (Sn), and lead (Pb), tend to be softer and less reactive than the transition metals. They demonstrate a lower degree of metallic character compared to those on the far left of the table, but still exhibit many typical metallic traits.
The Importance of Understanding Metallic Character
Understanding the periodic trends of metallic character is crucial in many scientific and technological applications:
- Material Science: The choice of materials for specific applications, like electrical wiring or structural components, heavily relies on their metallic properties. The knowledge of how metallic character changes across the periodic table guides the selection of appropriate materials for specific applications.
- Chemistry: The reactivity of elements, their ability to form compounds, and their behavior in chemical reactions are all influenced by their metallic or non-metallic character.
- Electronics: Semiconductors, which are often metalloids, play a vital role in modern electronics. Understanding their unique properties is essential for developing new electronic devices.
Conclusion: A More Nuanced Perspective
While the simple statement "metals are not on the right side of the periodic table" holds true as a general guideline, a complete understanding requires considering the complexities of periodic trends and the exceptions that exist. Metallic character is not a binary property; it's a spectrum, with elements showing varying degrees of metallic behavior across the periodic table. The location of an element on the table provides a valuable starting point for predicting its properties, but it is essential to account for the nuances and exceptions to develop a truly comprehensive understanding of metallic character and its significance in various fields. Further exploration of individual elements and their properties within their respective groups and periods will add deeper layers of understanding to this fascinating aspect of chemistry.
Latest Posts
Latest Posts
-
5 Steps Of The Listening Process
Mar 26, 2025
-
How To Determine If A Transformation Is Linear
Mar 26, 2025
-
How Do You Know If A Reaction Is Redox
Mar 26, 2025
-
Como Sacar El Diametro De Un Circulo
Mar 26, 2025
-
How To Find All Zeros Of A Polynomial
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Are Metals On The Right Side O The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
