Are Metals On The Right Side Of The Periodic Table
Muz Play
Mar 24, 2025 · 5 min read
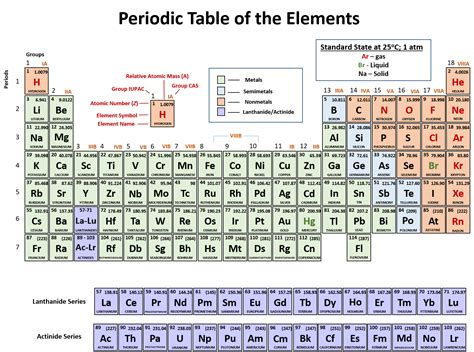
Table of Contents
Are Metals on the Right Side of the Periodic Table? Understanding Metallic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. One of the most fundamental classifications is the distinction between metals and nonmetals. While a quick glance might suggest metals are clustered on the left, the reality is more nuanced. This article delves deep into the fascinating world of metallic properties and their distribution across the periodic table, debunking the simplistic notion that metals exclusively reside on the left.
The Left-Right Dichotomy: A Simplified View
The common misconception that metals are solely located on the left side of the periodic table stems from a simplified representation often presented in introductory chemistry courses. This simplified view shows a clear division, with metals occupying the left and nonmetals the right. This is helpful for beginners to grasp the basic concept, but it lacks the complexity to fully represent the subtle variations in metallic character. The truth is far more intricate.
The Stair-Step Line: A More Accurate Depiction
A more accurate depiction utilizes a stair-step line separating metals from nonmetals. This line, running approximately from Boron (B) to Astatine (At), serves as a rough boundary. Elements to the left of this line generally exhibit metallic properties, while those to the right are typically nonmetals. However, even this representation oversimplifies the situation.
The Metalloids: Bridging the Gap
The elements bordering the stair-step line – Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, and Polonium – are known as metalloids or semimetals. These elements possess properties intermediate between metals and nonmetals, meaning they exhibit characteristics of both. This is why the simple left-right distinction fails; metalloids blur the lines completely, occupying a critical middle ground.
The Ambiguity of Metalloid Properties
Metalloids often exhibit semiconductivity, meaning their electrical conductivity lies somewhere between that of a conductor (metal) and an insulator (nonmetal). This unique characteristic makes them crucial components in modern electronics, particularly in semiconductors like transistors and integrated circuits. Their properties can also vary based on factors like temperature and pressure, adding another layer of complexity.
Exploring Metallic Trends Across the Periodic Table
Understanding the distribution of metals requires exploring trends in metallic properties. These trends are directly linked to atomic structure, specifically the number of valence electrons and atomic radius.
Valence Electrons and Metallic Bonding
Metals are characterized by their tendency to lose valence electrons relatively easily. This electron loss forms positive ions (cations) and contributes to the formation of metallic bonds. These bonds arise from the electrostatic attraction between the positively charged metal ions and the delocalized sea of electrons surrounding them. This "sea" of electrons explains metals' high electrical and thermal conductivity.
Atomic Radius and Metallic Character
Atomic radius, the size of an atom, also plays a significant role. Generally, as you move down a group (column) in the periodic table, the atomic radius increases. This increase leads to weaker attraction between the nucleus and valence electrons, making it easier for the electrons to be lost and thus enhancing metallic character. Conversely, moving across a period (row) from left to right, the atomic radius decreases, leading to a stronger hold on valence electrons and a decrease in metallic character.
Beyond the Simple Left-Right Division: Exceptions and Nuances
Several elements defy the simplistic left-right classification. Some metals, like Mercury (Hg), exist as liquids at room temperature, a property not always associated with typical metals. Furthermore, some elements considered metals exhibit properties that are less pronounced than those of the more strongly metallic elements.
Transition Metals: A Special Case
Transition metals, located in the d-block of the periodic table, represent a unique category. While undeniably metallic, they exhibit a broader range of properties compared to alkali or alkaline earth metals. Their ability to form multiple oxidation states, leading to colored compounds, adds to their complexity and challenges the simple left-right division.
Lanthanides and Actinides: The Inner Transition Metals
The lanthanides and actinides, situated at the bottom of the periodic table, represent another set of complexities. Their metallic character is undeniable, but their unique electronic configurations lead to specific properties, sometimes differing significantly from other metals. Their radioactive nature in the case of the actinides adds an additional layer of unique characteristics.
Factors Affecting Metallic Character
Several factors beyond simple position on the periodic table influence metallic properties:
-
Electronegativity: Elements with low electronegativity tend to be more metallic, as they have a weaker attraction for electrons.
-
Ionization Energy: Metals generally have low ionization energies, meaning it requires less energy to remove an electron.
-
Electrical Conductivity: Excellent electrical conductivity is a hallmark of metals, stemming from the delocalized electron sea.
-
Thermal Conductivity: High thermal conductivity is another key characteristic, reflecting the ease with which heat is transferred through the electron sea.
-
Malleability and Ductility: Metals are typically malleable (can be hammered into sheets) and ductile (can be drawn into wires), a result of the non-directional nature of metallic bonds.
The Importance of Understanding Metallic Trends
Understanding the nuanced distribution of metals and the factors influencing metallic character is crucial in several fields:
-
Materials Science: Designing new materials with specific properties often relies on the selection of metals with specific characteristics.
-
Chemistry: Predicting the reactivity and behavior of elements requires knowledge of their metallic nature.
-
Electronics: The semiconducting properties of metalloids are fundamental to modern electronics.
-
Metallurgy: The extraction and processing of metals rely on understanding their properties and behavior.
Conclusion: A More Nuanced Perspective
In conclusion, while the simplified left-right division of metals and nonmetals provides a basic understanding, it's crucial to adopt a more nuanced perspective. The presence of metalloids, the varied properties of transition metals and inner transition metals, and the influence of several atomic properties underscore the complexity of metallic character. The periodic table's organization provides a framework, but understanding the underlying trends and exceptions is essential for a thorough appreciation of the fascinating world of metallic elements and their distribution across the table. It is not simply a matter of "left" or "right," but a spectrum of properties reflecting intricate atomic structure and interactions.
Latest Posts
Latest Posts
-
Difference Between Substrate Level Phosphorylation And Oxidative Phosphorylation
Mar 26, 2025
-
5 Steps Of The Listening Process
Mar 26, 2025
-
How To Determine If A Transformation Is Linear
Mar 26, 2025
-
How Do You Know If A Reaction Is Redox
Mar 26, 2025
-
Como Sacar El Diametro De Un Circulo
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Are Metals On The Right Side Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
