Atom Equivalent To 7 Atoms Of Hydrogen
Muz Play
Mar 27, 2025 · 5 min read
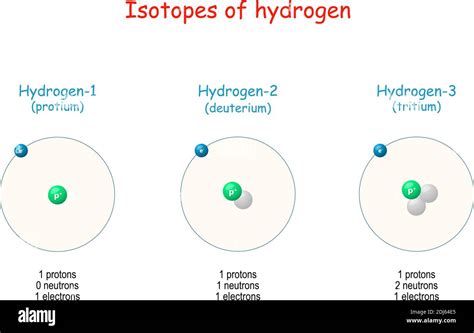
Table of Contents
Atom Equivalent to 7 Atoms of Hydrogen: Understanding Atomic Mass and Equivalence
The concept of an atom being "equivalent" to 7 hydrogen atoms isn't about a direct, physical substitution. Instead, it refers to atomic mass, a crucial concept in chemistry. This article delves deep into understanding atomic mass, its calculation, its significance in stoichiometry, and how it leads to the notion of an atom being equivalent to a certain number of hydrogen atoms. We'll explore the practical implications of this concept and address common misconceptions.
Atomic Mass: The Foundation of Equivalence
Atomic mass, also known as atomic weight, represents the average mass of an atom of a particular element, relative to the mass of a carbon-12 atom (assigned a mass of exactly 12 unified atomic mass units or u). It's crucial to understand that this is an average mass because most elements exist as a mixture of isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This difference in neutron number leads to variations in atomic mass.
For example, chlorine exists naturally as a mixture of two isotopes: chlorine-35 (approximately 75.77%) and chlorine-37 (approximately 24.23%). The atomic mass of chlorine listed on the periodic table (approximately 35.45 u) is a weighted average reflecting the abundance of each isotope.
Calculating Atomic Mass:
The calculation of the weighted average atomic mass involves multiplying the mass of each isotope by its relative abundance and then summing the results:
Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + ...
For example, if an element has two isotopes:
- Isotope 1: Mass = 10 u, Abundance = 60% (0.60)
- Isotope 2: Mass = 12 u, Abundance = 40% (0.40)
Atomic Mass = (10 u × 0.60) + (12 u × 0.40) = 10.8 u
The Significance of the Hydrogen Atom
Hydrogen, with its single proton and single electron (and typically no neutrons), holds a unique position in the periodic table. Its atomic mass is approximately 1 u (more precisely, 1.00784 u). Due to its simple structure and low mass, hydrogen often serves as a reference point for comparing the masses of other atoms.
What Does "Equivalent to 7 Hydrogen Atoms" Mean?
When we say an atom is equivalent to 7 hydrogen atoms, it means its atomic mass is approximately 7 times the atomic mass of hydrogen. Therefore, this atom would have an atomic mass of approximately 7 u. This is purely a comparison of relative mass; it doesn't imply that the atom is physically composed of seven hydrogen atoms.
Finding the Element:
Using the periodic table, we can search for an element with an atomic mass close to 7 u. While no element has an atomic mass precisely 7 u, Lithium (Li), with an atomic mass of approximately 6.94 u comes closest. Therefore, a Lithium atom can be considered approximately equivalent to 7 hydrogen atoms in terms of mass.
Practical Applications: Stoichiometry
The concept of atomic mass equivalence is fundamental to stoichiometry, the branch of chemistry dealing with the quantitative relationships between reactants and products in chemical reactions. It allows us to determine the relative amounts of substances involved in a reaction.
For example, consider a reaction between lithium and chlorine:
2Li + Cl₂ → 2LiCl
Based on the atomic masses, we know that one lithium atom is roughly equivalent to 7 hydrogen atoms, while one chlorine atom (approximately 35.45 u) is equivalent to roughly 35 hydrogen atoms. This allows us to calculate the mass ratios of reactants and products precisely.
Mole Concept and Avogadro's Number
The concept of atomic mass equivalence becomes even more powerful when combined with the mole concept and Avogadro's number (approximately 6.022 × 10²³). One mole of any substance contains Avogadro's number of elementary entities (atoms, molecules, ions, etc.). The mass of one mole of a substance is its molar mass, which is numerically equal to its atomic or molecular mass in grams.
Therefore, one mole of lithium (approximately 6.94 g) contains Avogadro's number of lithium atoms, each approximately equivalent to 7 hydrogen atoms in mass. Similarly, one mole of hydrogen (approximately 1 g) contains Avogadro's number of hydrogen atoms. This relationship allows precise calculations of the masses involved in reactions involving large numbers of atoms.
Beyond Atomic Mass: Other Considerations
While atomic mass equivalence provides a useful approximation, it's essential to remember that it solely focuses on mass. It does not consider other crucial properties of atoms, such as:
- Chemical properties: The chemical behavior of an element depends on its electronic configuration and not just its mass. Lithium and an element with a similar atomic mass but different electron configuration will exhibit very different chemical properties.
- Nuclear properties: Isotopes of the same element may have different nuclear properties, affecting their radioactivity or stability.
- Physical properties: Various physical properties like density, melting point, and boiling point differ vastly even between elements with similar atomic mass.
Addressing Common Misconceptions
-
Physical Composition: It is crucial to emphasize that saying an atom is "equivalent" to 7 hydrogen atoms does not imply that it's literally made up of 7 hydrogen atoms. It is merely a comparison of their relative masses.
-
Exact Equivalence: The equivalence is approximate because atomic masses are weighted averages. No element will have a precise atomic mass of 7 u.
-
Chemical Behavior: Equivalence in mass does not imply similar chemical behavior. Elements with similar atomic mass may have drastically different chemical properties.
Conclusion
The statement that an atom is "equivalent to 7 atoms of hydrogen" is a concise way of expressing its relative atomic mass compared to hydrogen. This concept, firmly rooted in the principles of atomic mass and stoichiometry, forms the basis for numerous quantitative calculations in chemistry. Understanding this concept allows for accurate predictions of reactant and product masses in chemical reactions, making it a fundamental tool for chemists and anyone working with chemical processes. Remember, however, that this comparison of mass is only one aspect of understanding atomic properties and should not be oversimplified to imply similarity in all other properties. The concept of atomic mass equivalence is a powerful tool when applied correctly and with awareness of its limitations.
Latest Posts
Latest Posts
-
What Is Held Constant In Gay Lussacs Law
Mar 31, 2025
-
Example Of A Line In A Poem
Mar 31, 2025
-
Interval Of Convergence Of A Taylor Series
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Atom Equivalent To 7 Atoms Of Hydrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
