Atomic Mass Equals The Number Of
Muz Play
Mar 26, 2025 · 6 min read
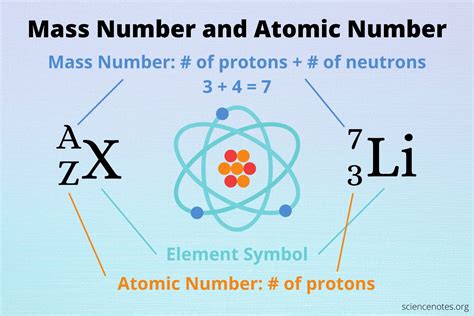
Table of Contents
Atomic Mass: More Than Just the Number of Protons
Atomic mass, also known as atomic weight, is a crucial concept in chemistry and physics. While it's often simplified as "the number of protons," this statement is only partially true. Understanding atomic mass requires delving deeper into the composition of an atom and the intricacies of isotopes. This comprehensive guide will explore the nuances of atomic mass, explaining what it truly represents and how it's determined.
What is an Atom? A Quick Recap
Before diving into atomic mass, let's briefly revisit the structure of an atom. An atom is the fundamental building block of matter, composed of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and determines its chemical properties.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. They participate in chemical bonding and determine the atom's reactivity.
Atomic Number vs. Atomic Mass: Key Differences
The atomic number is a whole number representing the number of protons in an atom's nucleus. This number uniquely identifies an element on the periodic table. For example, hydrogen has an atomic number of 1, helium has 2, and so on.
Atomic mass, however, is slightly more complex. It's the average mass of all the isotopes of an element, weighted by their relative abundance in nature. It's not simply the number of protons, but rather a reflection of the total number of protons and neutrons in the nucleus.
Isotopes: The Source of Atomic Mass Variation
The key to understanding the difference between atomic number and atomic mass lies in the concept of isotopes. Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This variation in neutron number leads to different mass numbers.
For example, carbon has three naturally occurring isotopes:
- Carbon-12 (¹²C): 6 protons and 6 neutrons (mass number = 12)
- Carbon-13 (¹³C): 6 protons and 7 neutrons (mass number = 13)
- Carbon-14 (¹⁴C): 6 protons and 8 neutrons (mass number = 14)
Notice that all three are carbon atoms because they all possess 6 protons. However, they differ in their neutron count and, consequently, their mass. The mass number is the sum of protons and neutrons in an individual atom's nucleus.
Calculating Atomic Mass: A Weighted Average
The atomic mass listed on the periodic table isn't the mass of a single isotope. Instead, it's a weighted average of the masses of all naturally occurring isotopes of that element. The weighting accounts for the relative abundance of each isotope.
Let's illustrate with carbon:
Assume the natural abundance of ¹²C is 98.9%, ¹³C is 1.1%, and ¹⁴C is negligible (its abundance is too small to significantly affect the average). To calculate the atomic mass of carbon:
(0.989 x 12 amu) + (0.011 x 13 amu) ≈ 12.01 amu
Where "amu" stands for atomic mass unit, a unit of mass approximately equal to the mass of a proton or neutron. Therefore, the atomic mass of carbon reported on the periodic table is approximately 12.01 amu.
Factors Affecting Atomic Mass
Several factors contribute to the complexity of determining atomic mass:
- Isotopic Abundance: The relative proportions of different isotopes in a naturally occurring sample of an element greatly influence the weighted average. Variations in isotopic abundance can exist depending on the sample's origin and geological history.
- Mass Spectrometer Measurements: Precise measurement of isotopic masses and their abundances is crucial for accurate atomic mass calculations. Mass spectrometry is the primary technique used to determine these values.
- Nuclear Binding Energy: While the mass number represents the sum of protons and neutrons, the actual mass of an atom is slightly less than the sum of the individual masses of its constituent particles. This difference is due to the release of energy (nuclear binding energy) when the nucleus forms. This energy loss translates to a slight mass defect, often accounted for using Einstein's famous equation, E=mc².
Atomic Mass in Chemical Calculations
Atomic mass plays a critical role in various chemical calculations, including:
- Molar Mass Calculations: The molar mass of a substance is the mass of one mole (6.022 x 10²³ particles) of that substance. It's calculated using the atomic masses of the constituent elements.
- Stoichiometric Calculations: Atomic mass is essential in stoichiometry, allowing us to convert between the mass of a substance and the number of moles, enabling accurate predictions of reactant and product amounts in chemical reactions.
- Determining Empirical and Molecular Formulas: Atomic mass helps determine the empirical and molecular formulas of compounds by analyzing the relative masses of elements in the compound.
Applications of Atomic Mass Knowledge
Understanding atomic mass extends beyond textbook chemistry. It has practical applications in various fields:
- Nuclear Medicine: Isotopes with specific atomic masses are used in diagnostic and therapeutic nuclear medicine procedures. For example, ¹³¹I is employed in thyroid cancer treatment.
- Radioactive Dating: The decay rates of specific isotopes (e.g., ¹⁴C) are used in radiocarbon dating to determine the age of ancient artifacts and organic materials.
- Geochemistry: Isotopic ratios in geological samples provide insights into Earth's processes and formation history.
- Forensic Science: Isotopic analysis can be used in forensic science to trace the origin of materials and substances.
Beyond the Basics: Advanced Concepts
While we've covered the fundamental aspects of atomic mass, more advanced concepts exist:
- Relative Atomic Mass: This term is often used synonymously with atomic weight, emphasizing that it is a relative measure compared to a standard (usually ¹²C).
- Standard Atomic Weight: The International Union of Pure and Applied Chemistry (IUPAC) periodically updates standard atomic weights for elements, reflecting the most accurate and up-to-date isotopic abundance data.
- Average Atomic Mass vs. Mass Number: It's crucial to distinguish between the average atomic mass (a weighted average) and the mass number (the sum of protons and neutrons for a specific isotope).
Conclusion: Atomic Mass – A Comprehensive Understanding
Atomic mass is far more intricate than simply the number of protons. It's a weighted average reflecting the masses and abundances of an element's isotopes, a concept deeply rooted in the structure of the atom and its subatomic particles. Understanding atomic mass is foundational to numerous chemical calculations and has widespread applications in various scientific disciplines. By grasping the nuances of isotopes, isotopic abundance, and the process of calculating atomic mass, one gains a deeper appreciation for the complexities and subtleties of the atomic world. This understanding is essential for anyone pursuing studies in chemistry, physics, or related fields. Moreover, the continuous refinement of atomic mass measurements underscores the ongoing evolution of scientific knowledge and its constant adaptation to new discoveries and advancements in analytical techniques.
Latest Posts
Latest Posts
-
Genomics Can Be Used In Agriculture To
Mar 29, 2025
-
Standard Enthalpy Of Formation For O2
Mar 29, 2025
-
How To Determine If A Reaction Is Spontaneous
Mar 29, 2025
-
What Bonds Are The Most Polar
Mar 29, 2025
-
Systems Of Linear Equations And Inequalities
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Atomic Mass Equals The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
