Standard Enthalpy Of Formation For O2
Muz Play
Mar 29, 2025 · 6 min read
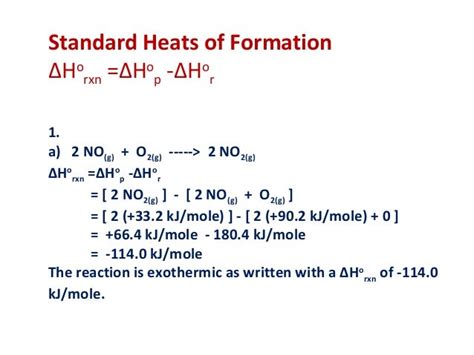
Table of Contents
Standard Enthalpy of Formation for O₂: A Deep Dive
The standard enthalpy of formation, often denoted as ΔfH°, represents the change in enthalpy during the formation of one mole of a substance from its constituent elements in their standard states. Understanding this concept is fundamental to thermochemistry and crucial for various applications in chemistry and engineering. This article will delve into the specific case of O₂, diatomic oxygen, exploring why its standard enthalpy of formation is zero, its implications, and its role in broader thermodynamic calculations.
The Standard State and its Significance
Before we delve into the specifics of O₂, let's clarify the concept of the "standard state." This refers to a set of precisely defined conditions used as a reference point for thermodynamic calculations. For gases, the standard state is defined as a pressure of 1 atmosphere (atm) or 101.325 kilopascals (kPa). For pure substances in condensed phases (solids or liquids), the standard state is the pure substance at 1 atm pressure. The standard temperature is universally accepted as 298.15 Kelvin (K), or 25 degrees Celsius (°C). These standard conditions allow for consistent comparison of thermodynamic properties across different reactions and substances.
Why ΔfH°(O₂) = 0
The standard enthalpy of formation of O₂(g) is zero, a fact that often causes initial confusion. This stems directly from the definition of standard enthalpy of formation. The definition explicitly states that the enthalpy change is measured for the formation of one mole of a substance from its constituent elements in their standard states. In the case of O₂, the constituent element is oxygen itself. Since O₂ is already the standard state of oxygen at 298.15 K and 1 atm, the formation reaction becomes:
O₂(g) → O₂(g)
There is no change in the system. No chemical reaction occurs; therefore, no enthalpy change is involved. Consequently, the standard enthalpy of formation is zero. This isn't an exception to the rule; it's a direct consequence of the definition itself. Any element in its standard state will have a standard enthalpy of formation of zero. This includes:
- Carbon (graphite): ΔfH°(C(graphite)) = 0 kJ/mol
- Hydrogen (H₂): ΔfH°(H₂(g)) = 0 kJ/mol
- Nitrogen (N₂): ΔfH°(N₂(g)) = 0 kJ/mol
Implications and Applications
The fact that ΔfH°(O₂(g)) = 0 is crucial for calculating the standard enthalpy changes (ΔrH°) of various chemical reactions involving oxygen. The standard enthalpy change of a reaction can be calculated using Hess's Law, which states that the enthalpy change of a reaction is independent of the pathway taken. Hess's Law is elegantly expressed using standard enthalpies of formation:
ΔrH° = Σ [ΔfH°(products)] - Σ [ΔfH°(reactants)]
This equation highlights the importance of the standard enthalpies of formation of all reactants and products. Since the standard enthalpy of formation of O₂ is zero, it simplifies the calculation for many combustion and oxidation reactions where oxygen is a reactant. Without this zero value, the calculations would be significantly more complex.
Beyond Standard Conditions: Temperature and Pressure Dependence
While the standard enthalpy of formation is defined at standard conditions (298.15 K and 1 atm), the enthalpy of formation is actually temperature and pressure-dependent. To calculate the enthalpy of formation at different conditions, we can use thermodynamic relationships like Kirchhoff's Law. Kirchhoff's Law relates the change in enthalpy with temperature:
ΔCp = Σ Cp(products) - Σ Cp(reactants)
Where ΔCp represents the difference in heat capacity at constant pressure between products and reactants. By integrating this equation over a temperature range, we can calculate the change in enthalpy of formation between different temperatures. However, the pressure dependence is often less significant than the temperature dependence, especially for condensed phases.
Accuracy and Uncertainty
The value of ΔfH°(O₂(g)) = 0 is an idealized value based on the definition. In reality, there will be slight variations due to factors such as isotopic composition and experimental uncertainties in measuring thermodynamic properties. However, these variations are typically negligible for most practical purposes. Precise values of standard enthalpies of formation are usually accompanied by uncertainty ranges to reflect the inherent experimental errors.
Standard Enthalpy of Formation in Other Applications
The concept of standard enthalpy of formation extends far beyond simply calculating reaction enthalpies. It plays a crucial role in:
- Predicting reaction spontaneity: Combined with entropy changes, the standard enthalpy of formation allows the calculation of Gibbs free energy (ΔG°), which determines the spontaneity of a reaction.
- Industrial process design: The enthalpy of formation is crucial in designing and optimizing industrial processes, such as combustion engines and chemical synthesis, to maximize efficiency and minimize energy consumption.
- Material science: Understanding the enthalpies of formation of different materials is critical in the design and development of new materials with specific properties.
- Environmental chemistry: The enthalpy of formation is used to model and predict the thermodynamics of environmental processes, such as atmospheric reactions and pollutant degradation.
Understanding the Zero Point: A Deeper Look
The zero value for ΔfH°(O₂) isn’t merely a convenient number; it reflects a fundamental aspect of thermodynamics. It's a consequence of choosing the elements in their standard states as the reference point for enthalpy. We could, theoretically, choose a different reference point, and the enthalpies of formation would change accordingly, but the relative values and the overall consistency of thermodynamic calculations would remain unchanged. This highlights the importance of clearly defining reference points in thermodynamic calculations.
Beyond O₂: Exploring Other Elements and Compounds
While O₂ is a critical example because of its prevalence in many chemical reactions, the principle of zero standard enthalpy of formation applies to all elements in their standard states. For compounds, however, the standard enthalpy of formation is non-zero and reflects the energy involved in forming chemical bonds from their constituent elements. Understanding these values for various compounds is equally critical for various thermodynamic calculations. Databases of standard enthalpy of formation values are readily available for a wide range of substances.
Conclusion: A Foundational Concept in Thermochemistry
The standard enthalpy of formation for O₂, while seemingly simple at first glance, holds significant importance within the broader context of thermochemistry. Its zero value isn't an anomaly; it's a direct consequence of the definition and a cornerstone for numerous calculations and applications across various scientific and engineering disciplines. By understanding this fundamental concept and its implications, we gain a deeper appreciation for the power and elegance of thermodynamics in understanding and predicting the behavior of chemical systems. Its role in simplifying calculations and providing a consistent framework for comparing different reactions underscores its vital role in chemical thermodynamics and its many applications.
Latest Posts
Latest Posts
-
How Many Protons Are In A Sulfur Atom
Mar 31, 2025
-
How Many Bases In A Codon
Mar 31, 2025
-
Glucose And Galactose Differ At Which Carbon
Mar 31, 2025
-
Write The Formulas For The Following Compounds
Mar 31, 2025
-
Electric Field Due To Ring Of Charge
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Standard Enthalpy Of Formation For O2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
