Glucose And Galactose Differ At Which Carbon
Muz Play
Mar 31, 2025 · 5 min read
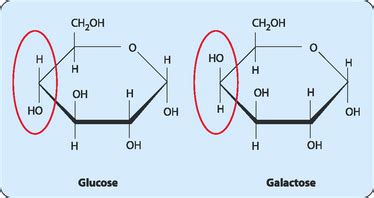
Table of Contents
Glucose and Galactose Differ at Carbon 4: A Deep Dive into Monosaccharide Structure and Function
Understanding the subtle differences between seemingly similar molecules is crucial in biochemistry. Glucose and galactose, both hexose monosaccharides, are prime examples. While they share the same chemical formula (C₆H₁₂O₆), a single difference in their structure dramatically impacts their properties and biological roles. This article will explore the key structural difference between glucose and galactose, focusing on the significance of the differing carbon atom and its consequences for their metabolism and function in the body.
The Structural Distinction: A Matter of Stereochemistry
Both glucose and galactose are aldohexoses, meaning they are six-carbon sugars with an aldehyde group at carbon 1. The key difference lies in the stereochemistry at carbon 4. To understand this, let's delve into the Fischer projections:
Fischer Projections: Visualizing the Difference
Fischer projections provide a simplified 2D representation of the 3D structure of monosaccharides. In a Fischer projection, the vertical lines represent bonds projecting away from the viewer, and horizontal lines represent bonds projecting towards the viewer.
Glucose: In the D-glucose Fischer projection (the most biologically relevant form), the hydroxyl (-OH) group at carbon 4 is pointing to the right.
Galactose: In the D-galactose Fischer projection, the hydroxyl (-OH) group at carbon 4 is pointing to the left.
This seemingly minor difference in the orientation of a single hydroxyl group leads to significant differences in their three-dimensional shapes and subsequent biological behavior. This difference at C4 is the defining characteristic that distinguishes these two important monosaccharides.
Implications of the C4 Difference: Beyond Simple Structure
The alteration at carbon 4 significantly affects several properties of galactose compared to glucose, impacting its reactivity, metabolism, and biological function.
1. Different Conformations: Cyclization and Anomeric Forms
In aqueous solutions, glucose and galactose predominantly exist in cyclic forms, primarily as pyranose rings (six-membered rings). The cyclization process involves the reaction of the aldehyde group at carbon 1 with the hydroxyl group at carbon 5. This cyclization introduces a new chiral center at carbon 1, creating α and β anomers. While both glucose and galactose form both α and β anomers, the different configuration at carbon 4 influences the equilibrium between these anomers and their overall conformational preferences. These subtle differences in conformation affect their interactions with enzymes and other molecules.
2. Metabolic Pathways: Distinct Enzymatic Recognition
The unique three-dimensional structure resulting from the C4 difference is crucial for enzyme recognition. Enzymes are highly specific, often binding only to molecules with a precise shape and stereochemistry. Because of this difference, glucose and galactose are metabolized through distinct pathways.
Glucose Metabolism: Glucose is the primary fuel source for most cells. It enters glycolysis, the central pathway of carbohydrate metabolism, through a series of well-characterized enzymatic reactions.
Galactose Metabolism: Galactose requires a separate metabolic pathway, the Leloir pathway, for conversion to glucose. This pathway involves several specific enzymes that recognize and act upon the unique configuration of galactose. The initial step involves the conversion of galactose to galactose-1-phosphate, which is subsequently converted to glucose-1-phosphate and finally to glucose-6-phosphate, allowing entry into the glycolytic pathway. Defects in enzymes within the Leloir pathway can lead to galactosemia, a serious genetic disorder.
3. Glycosidic Linkages and Polysaccharide Formation
Both glucose and galactose can participate in the formation of glycosidic linkages, which connect monosaccharides to form disaccharides, oligosaccharides, and polysaccharides. However, the specific glycosidic linkages formed will differ depending on the configuration at carbon 4. This leads to the formation of different polysaccharides with distinct properties and biological roles. For instance, glucose forms cellulose, a major structural component of plant cell walls, whereas galactose is a component of galactomannans, found in various plant seeds. The difference at carbon 4 directly influences the overall structure and function of these polysaccharides.
4. Interactions with Receptors and Other Biomolecules
The three-dimensional structure, dictated in part by the C4 configuration, influences the interaction of glucose and galactose with various receptors and other biomolecules. For example, certain cell surface receptors exhibit specific binding affinities for glucose versus galactose, leading to different biological responses. This specificity in binding is crucial for processes like cell signaling and nutrient uptake.
Clinical Significance: Galactosemia and the Importance of C4
As mentioned earlier, differences in galactose metabolism, stemming from the C4 difference, have significant clinical implications. Galactosemia is a group of inherited metabolic disorders resulting from deficiencies in enzymes of the Leloir pathway. These deficiencies prevent the efficient conversion of galactose to glucose, leading to the accumulation of galactose and its derivatives in the blood and tissues. This accumulation can cause severe health problems, including liver damage, cataracts, and intellectual disability. Early diagnosis and dietary management are crucial for mitigating the effects of galactosemia. This highlights the profound impact of the seemingly small structural difference between glucose and galactose on human health.
Conclusion: The Power of a Single Carbon
The seemingly insignificant difference in the orientation of the hydroxyl group at carbon 4 dramatically affects the properties and biological roles of glucose and galactose. This single structural variation leads to differences in:
- Conformation and cyclization: affecting enzyme binding and reactivity.
- Metabolic pathways: requiring distinct enzymatic processes for metabolism.
- Glycosidic linkages: leading to diverse polysaccharide structures and functions.
- Interactions with receptors: impacting cellular responses and signaling.
Understanding this difference is critical for comprehending carbohydrate metabolism, the pathophysiology of metabolic disorders like galactosemia, and the diverse roles of these monosaccharides in biological systems. The seemingly minor change at carbon 4 underscores the remarkable power of stereochemistry in shaping the functions of biological molecules. Further research into the subtleties of these structural differences will undoubtedly continue to unveil even more nuanced aspects of their biological significance. The focus on the C4 difference serves as an excellent illustration of the intricate relationship between molecular structure and biological function.
Latest Posts
Latest Posts
-
How To Make Normal Probability Plot
Apr 02, 2025
-
Chemical Kinetics Of The Iodine Clock Reaction Lab Report
Apr 02, 2025
-
Who Is Credited With Discovering Cells
Apr 02, 2025
-
Calculate The Ph At The Equivalence Point For The Titration
Apr 02, 2025
-
Gramatica A The Verb Tener Answers
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Glucose And Galactose Differ At Which Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
