How Many Protons Are In A Sulfur Atom
Muz Play
Mar 31, 2025 · 5 min read
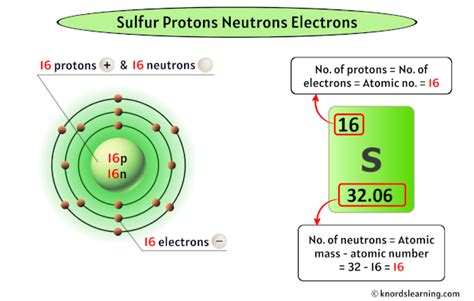
Table of Contents
How Many Protons are in a Sulfur Atom? Understanding Atomic Structure and Sulfur's Properties
Determining the number of protons in a sulfur atom is fundamental to understanding its chemical behavior and place within the periodic table. This article delves into the atomic structure of sulfur, explaining how to identify its proton count, and exploring the implications of this number for its various properties and applications. We'll also touch upon related concepts like isotopes and ions, expanding our understanding of this essential element.
Understanding Atomic Structure: The Foundation of Chemistry
Before diving into sulfur specifically, let's establish a firm grasp of basic atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles residing within the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutral particles (no charge) also found in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The nucleus, containing the protons and neutrons, accounts for almost all the mass of an atom. The electrons, while significantly contributing to chemical reactions, possess negligible mass compared to the nucleus.
Identifying Sulfur on the Periodic Table
The periodic table is a chemist's roadmap, systematically organizing elements based on their atomic number and properties. Each element occupies a unique square, containing crucial information, including its atomic number. Sulfur (S) is located in period 3, group 16 (or VIA) of the periodic table. Its atomic number, prominently displayed, is 16.
The Answer: Sulfur's Proton Count
This atomic number of 16 directly tells us the number of protons in a sulfur atom. Therefore, a sulfur atom contains 16 protons. This is a fundamental characteristic that distinguishes sulfur from all other elements. No other element possesses 16 protons.
Isotopes of Sulfur: Variations in Neutron Count
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Sulfur has several naturally occurring isotopes, including:
- Sulfur-32 (³²S): The most abundant isotope, containing 16 protons and 16 neutrons.
- Sulfur-33 (³³S): A less abundant isotope, with 16 protons and 17 neutrons.
- Sulfur-34 (³⁴S): Another naturally occurring isotope, having 16 protons and 18 neutrons.
- Sulfur-36 (³⁶S): A less abundant, stable isotope with 16 protons and 20 neutrons.
The mass number (shown as a superscript before the element symbol) represents the total number of protons and neutrons in an atom's nucleus. For example, ³²S has a mass number of 32 (16 protons + 16 neutrons). These isotopic variations do not alter the chemical properties significantly, but they do affect the average atomic mass of sulfur, which is a weighted average reflecting the abundance of each isotope.
Ions of Sulfur: Gaining or Losing Electrons
Atoms can gain or lose electrons to form ions. Ions are charged species because the number of protons and electrons is no longer equal. Sulfur, being in Group 16, often gains two electrons to achieve a stable octet configuration (eight electrons in its outermost shell). This forms the sulfide ion (S²⁻), which now has 16 protons and 18 electrons, resulting in a net charge of -2.
The Significance of Sulfur's 16 Protons: Properties and Applications
The presence of 16 protons dictates sulfur's chemical and physical properties, leading to a wide range of applications:
Chemical Properties:
- Reactivity: Sulfur's six valence electrons (electrons in the outermost shell) make it relatively reactive, readily forming covalent bonds with other elements. This contributes to its ability to form various compounds, including sulfides, sulfates, and sulfur oxides.
- Oxidation States: Sulfur can exhibit various oxidation states, ranging from -2 (in sulfides) to +6 (in sulfates), depending on the bonding partner. This versatility contributes to its role in numerous chemical reactions.
- Acidity: Many sulfur-containing compounds exhibit acidic properties, influencing their environmental impact and industrial applications. Sulfuric acid (H₂SO₄), for instance, is a strong acid widely used in various industrial processes.
Physical Properties:
- Appearance: Elemental sulfur is a yellow, crystalline solid at room temperature.
- Melting and Boiling Points: Sulfur has relatively low melting and boiling points compared to many other elements, due to weaker intermolecular forces.
- Electrical Conductivity: Elemental sulfur is a poor conductor of electricity, highlighting its non-metallic character.
Applications of Sulfur and its Compounds:
Sulfur's diverse properties have led to its widespread use in many industries:
- Vulcanization of Rubber: Sulfur is crucial in vulcanizing rubber, creating stronger and more elastic materials used in tires, hoses, and other products.
- Sulfuric Acid Production: Sulfuric acid, the most produced chemical globally, is a cornerstone of many industries, including fertilizers, detergents, and metal processing.
- Fertilizers: Sulfur is an essential nutrient for plant growth, and sulfur-containing fertilizers play a vital role in agriculture.
- Pharmaceuticals: Certain sulfur-containing compounds find application in pharmaceuticals, contributing to medicinal properties.
- Matches and Fireworks: Sulfur is a key component in the manufacture of matches and fireworks due to its flammability.
- Metal Extraction: Sulfur is used in the extraction of certain metals from their ores.
Conclusion: The Central Role of Protons in Defining Sulfur
The number of protons in a sulfur atom—16—is not just a number; it’s the defining characteristic of this element. This number dictates its position on the periodic table, determines its chemical and physical properties, and ultimately shapes its vast array of applications across various industries. Understanding atomic structure and the significance of the atomic number is fundamental to comprehending the behavior of all elements, including the vital role of sulfur in our world. From its presence in essential biological molecules to its importance in industrial processes, sulfur's unique properties are a direct consequence of its 16 protons. The study of sulfur's atomic structure provides a clear illustration of the powerful link between atomic-level characteristics and macroscopic properties. This connection underscores the essential role of chemistry in understanding the world around us.
Latest Posts
Latest Posts
-
Area Between Two Polar Curves Formula
Apr 02, 2025
-
A Bronsted Lowry Base Is Defined As
Apr 02, 2025
-
Molecular And Empirical Formula Worksheet With Answers
Apr 02, 2025
-
How To Find The Maclaurin Series
Apr 02, 2025
-
How To Make Normal Probability Plot
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Are In A Sulfur Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
