Atomic Structure Of Elements In Periodic Table
Muz Play
Mar 29, 2025 · 7 min read
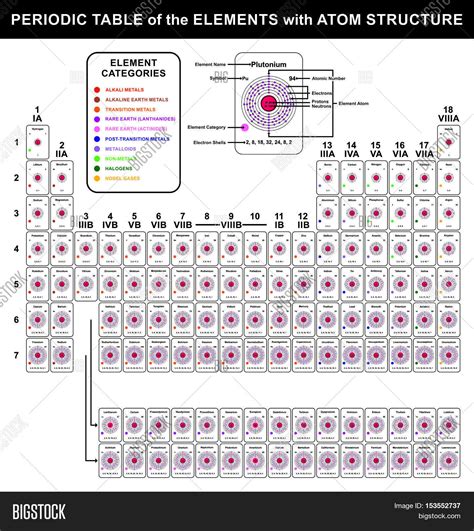
Table of Contents
Delving Deep: The Atomic Structure of Elements in the Periodic Table
The periodic table, a seemingly simple grid of elements, actually represents a profound understanding of the fundamental building blocks of matter: atoms. Understanding the atomic structure of elements is key to comprehending their properties, behavior, and how they interact to form the diverse materials that make up our universe. This article provides a comprehensive exploration of atomic structure, its relation to the periodic table, and the implications for chemistry and beyond.
The Fundamental Particles: A Foundation for Understanding
Before diving into the complexities of atomic structure, let's establish a foundation by defining the three fundamental subatomic particles:
1. Protons: The Positive Charge Carriers
Protons reside within the atom's nucleus, carrying a single positive electrical charge (+1). The number of protons in an atom's nucleus defines its atomic number, and this number uniquely identifies the element. For instance, an atom with one proton is hydrogen (atomic number 1), while an atom with six protons is carbon (atomic number 6). Protons contribute significantly to the atom's mass.
2. Neutrons: The Neutral Partners
Also located in the nucleus, neutrons possess no electrical charge (neutral). Their primary role is to contribute to the atom's mass. Isotopes, variations of an element with different numbers of neutrons, exist because the number of neutrons can vary without altering the element's identity (its atomic number). For example, carbon-12 and carbon-14 are isotopes of carbon; both have six protons, but carbon-12 has six neutrons while carbon-14 has eight.
3. Electrons: The Orbiting Negatives
Electrons are significantly smaller and lighter than protons and neutrons and occupy the space surrounding the nucleus. They carry a single negative electrical charge (-1). The movement of electrons is key to many chemical and physical properties of elements. Electrons are arranged in energy levels or shells, each capable of holding a specific number of electrons. These electron arrangements are crucial in determining how atoms interact with each other, forming chemical bonds.
Electron Shells and Subshells: Unveiling the Electron Configuration
Electrons don't orbit the nucleus randomly; they occupy specific energy levels called shells. Each shell corresponds to a principal quantum number (n), with n = 1 representing the shell closest to the nucleus, n = 2 the next, and so on. As 'n' increases, the energy level of the shell increases, and the electrons are further from the nucleus.
Within each shell are subshells, which are designated by letters (s, p, d, f). Each subshell can hold a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The electron configuration of an element describes how its electrons are distributed among the shells and subshells. For example, the electron configuration of oxygen (atomic number 8) is 1s²2s²2p⁴, indicating that two electrons occupy the 1s subshell, two occupy the 2s subshell, and four occupy the 2p subshell.
The Periodic Table: A Visual Representation of Atomic Structure
The periodic table organizes elements based on their atomic number and electron configuration. Elements in the same column (group) have similar electron configurations in their outermost shell (valence shell), resulting in similar chemical properties. For instance, all elements in Group 1 (alkali metals) have one electron in their valence shell, leading to their high reactivity. Elements in the same row (period) have the same number of electron shells.
Understanding Trends in the Periodic Table
The arrangement of the periodic table reflects trends in various atomic properties, including:
- Atomic Radius: Generally increases down a group and decreases across a period.
- Ionization Energy: The energy required to remove an electron from an atom. Generally decreases down a group and increases across a period.
- Electronegativity: A measure of an atom's ability to attract electrons in a chemical bond. Generally decreases down a group and increases across a period.
- Electron Affinity: The energy change when an electron is added to a neutral atom.
These trends are directly linked to the atomic structure of the elements, particularly the number of electrons in the valence shell and the effective nuclear charge experienced by the outermost electrons.
Beyond Basic Atomic Structure: Isotopes and Ions
The simple model of atomic structure described above needs expansion to incorporate isotopes and ions:
Isotopes: Variations on a Theme
As mentioned earlier, isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties. Many elements exist as mixtures of different isotopes. For example, naturally occurring carbon is a mixture of carbon-12 (98.9%) and carbon-13 (1.1%), with trace amounts of carbon-14.
Ions: Charged Particles
Ions are atoms or molecules that have gained or lost electrons, resulting in a net electrical charge. Cations are positively charged ions (formed by losing electrons), while anions are negatively charged ions (formed by gaining electrons). The formation of ions is crucial in many chemical reactions and the formation of ionic compounds. For example, sodium (Na) readily loses one electron to form a sodium cation (Na⁺), while chlorine (Cl) readily gains one electron to form a chloride anion (Cl⁻). The electrostatic attraction between these oppositely charged ions leads to the formation of sodium chloride (NaCl), table salt.
Atomic Orbitals: A Quantum Mechanical Perspective
The simple shell model of atomic structure provides a useful framework, but a more accurate description requires quantum mechanics. Quantum mechanics describes electrons not as particles orbiting the nucleus in neat circular paths, but as existing in orbitals – regions of space where there is a high probability of finding an electron.
Each orbital is characterized by a set of quantum numbers:
- Principal quantum number (n): Determines the energy level and size of the orbital.
- Azimuthal quantum number (l): Determines the shape of the orbital (s, p, d, f).
- Magnetic quantum number (ml): Determines the orientation of the orbital in space.
- Spin quantum number (ms): Describes the intrinsic angular momentum of the electron (spin up or spin down).
The shapes of atomic orbitals are complex and vary depending on the values of n and l. s orbitals are spherical, p orbitals are dumbbell-shaped, and d and f orbitals have more complex shapes.
The Significance of Atomic Structure in Chemistry and Beyond
Understanding the atomic structure of elements is fundamental to all areas of chemistry:
- Chemical Bonding: The way atoms interact to form molecules and compounds is directly governed by their electron configurations and the tendency to achieve stable electron arrangements (usually a full valence shell).
- Chemical Reactions: Chemical reactions involve the rearrangement of atoms and electrons, and understanding atomic structure helps predict the outcome of reactions.
- Materials Science: The properties of materials depend heavily on the atomic structure and bonding of the constituent elements.
- Nuclear Chemistry: This branch of chemistry deals with the nucleus of the atom, including radioactive decay and nuclear reactions.
Furthermore, atomic structure has implications far beyond chemistry:
- Physics: Understanding atomic structure is vital in various fields of physics, including nuclear physics, particle physics, and condensed matter physics.
- Biology: The structure and function of biological molecules, such as proteins and DNA, are determined by the atomic structure of their constituent elements.
- Medicine: Medical imaging techniques, such as MRI and PET scans, rely on the interaction of atomic nuclei with magnetic fields and radiation.
- Engineering: The design and development of new materials and technologies often require a deep understanding of atomic structure and its influence on material properties.
Conclusion: A Continuing Journey of Discovery
The atomic structure of elements, as revealed through the periodic table and advancements in quantum mechanics, represents a monumental achievement in scientific understanding. This knowledge forms the cornerstone of chemistry and has far-reaching implications across numerous scientific disciplines and technological advancements. While our understanding continues to evolve, the fundamental principles laid out in this exploration remain crucial for comprehending the world around us and pushing the boundaries of scientific discovery. The journey into the atom's intricacies is a testament to human curiosity and our persistent quest to unravel the secrets of the universe.
Latest Posts
Latest Posts
-
Abstract Algebra Theory And Applications Judson
Apr 01, 2025
-
What Is The Second Stage Of Cellular Respiration
Apr 01, 2025
-
What Is The Basic Structural Unit Of The Body
Apr 01, 2025
-
Motion Of Molecules Compared To Direction Of Motion Electromagnetic Waves
Apr 01, 2025
-
The Variance Is The Square Root Of The Standard Deviation
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Atomic Structure Of Elements In Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
