Atoms In Molecules Share Pairs Of Electrons When They Make
Muz Play
Mar 17, 2025 · 6 min read
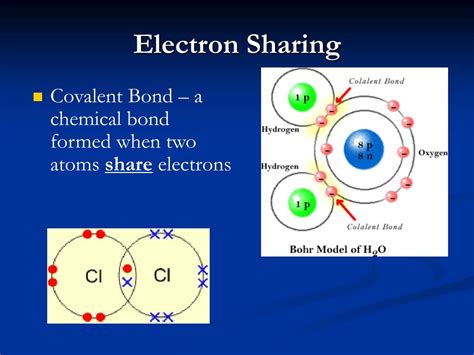
Table of Contents
Atoms in Molecules Share Pairs of Electrons When They Make Covalent Bonds: A Deep Dive
The fundamental building blocks of matter, atoms, constantly interact to form the molecules that make up our world. One of the most crucial ways atoms interact is through the sharing of electrons, a process that forms the bedrock of covalent bonds. This article will delve deep into the intricacies of covalent bonding, exploring the mechanisms behind electron sharing, the types of covalent bonds, and the impact this bonding has on the properties of molecules.
Understanding Atomic Structure and Electron Configuration
Before we dive into electron sharing, it's crucial to understand the basic structure of an atom. Atoms consist of a nucleus, containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons orbiting in specific energy levels or shells. The outermost shell, known as the valence shell, plays a pivotal role in chemical bonding. Atoms strive for a stable electron configuration, often achieving this by having a full valence shell, a principle often referred to as the octet rule (eight electrons). This drive for stability dictates how atoms interact and form bonds.
The Octet Rule and its Exceptions
While the octet rule provides a useful framework for understanding covalent bonding, it's essential to acknowledge its exceptions. Some atoms, particularly those in the second period (like hydrogen, lithium, beryllium, and boron), can form stable molecules with fewer than eight electrons in their valence shell. Elements like phosphorus and sulfur can also expand their octet, accommodating more than eight electrons in their valence shell, particularly when bonding with highly electronegative atoms like oxygen and fluorine. These exceptions highlight the complex nature of chemical bonding and underscore the need for a deeper understanding beyond simplistic rules.
Covalent Bonds: Sharing is Caring
Atoms achieve stable electron configurations by sharing electrons in their valence shells, forming covalent bonds. This sharing creates a strong attractive force between the atoms, holding them together to form molecules. The shared electrons are considered to be part of both atoms' valence shells, contributing to the stability of each.
The Nature of Shared Electron Pairs
The shared electron pairs in a covalent bond are not necessarily evenly distributed between the atoms involved. The electronegativity of an atom—its ability to attract electrons in a chemical bond—plays a crucial role. If the electronegativity of the two atoms is significantly different, the shared electrons are pulled closer to the more electronegative atom, resulting in a polar covalent bond. If the electronegativity difference is minimal, the electrons are shared more equally, resulting in a nonpolar covalent bond.
Polar Covalent Bonds: Unequal Sharing
In polar covalent bonds, the more electronegative atom carries a partial negative charge (δ-), while the less electronegative atom carries a partial positive charge (δ+). This charge separation creates a dipole moment, a vector quantity representing the magnitude and direction of the bond's polarity. Water (H₂O) is a classic example of a molecule with polar covalent bonds, leading to its unique properties as a solvent and its high boiling point.
Nonpolar Covalent Bonds: Equal Sharing
Nonpolar covalent bonds are characterized by an equal sharing of electrons between atoms of similar electronegativity. Diatomic molecules like oxygen (O₂) and nitrogen (N₂) are excellent examples of molecules with nonpolar covalent bonds. The symmetrical distribution of electrons leads to a balanced charge distribution across the molecule.
Types of Covalent Bonds
Covalent bonds are not monolithic; they exhibit variations in bond order and strength.
Single, Double, and Triple Bonds
The number of electron pairs shared between two atoms determines the bond order and influences the bond length and strength. A single bond involves one shared electron pair, a double bond involves two shared electron pairs, and a triple bond involves three shared electron pairs. Generally, triple bonds are shorter and stronger than double bonds, which are in turn shorter and stronger than single bonds. The increased electron density in multiple bonds leads to stronger attractions between the atoms.
Coordinate Covalent Bonds: A Special Case
A coordinate covalent bond, also known as a dative bond, is a special type of covalent bond where both electrons in the shared pair originate from the same atom. This often occurs when a molecule or ion with a lone pair of electrons (a Lewis base) donates its electrons to an atom or ion that needs electrons to complete its octet (a Lewis acid). The ammonium ion (NH₄⁺) is a classic example, where the nitrogen atom in ammonia (NH₃) donates a lone pair of electrons to a proton (H⁺).
Factors Influencing Covalent Bond Strength
Several factors influence the strength of a covalent bond:
- Bond Order: As mentioned earlier, higher bond orders (double and triple bonds) lead to stronger bonds.
- Atomic Size: Smaller atoms generally form stronger bonds because the shared electrons are closer to the positively charged nuclei.
- Electronegativity: While electronegativity differences lead to polarity, moderate differences can actually strengthen the bond due to increased electrostatic attraction. However, excessively large differences can weaken the bond.
Consequences of Covalent Bonding: Molecular Properties
The nature of covalent bonding significantly influences the physical and chemical properties of molecules:
- Melting and Boiling Points: Covalent compounds generally have lower melting and boiling points compared to ionic compounds because the intermolecular forces (forces between molecules) are weaker than the electrostatic forces in ionic compounds. However, the strength of intermolecular forces varies significantly depending on the polarity and size of the molecule.
- Solubility: The solubility of covalent compounds depends on the polarity of both the compound and the solvent. Polar covalent compounds tend to dissolve in polar solvents (like water), while nonpolar covalent compounds dissolve in nonpolar solvents (like organic solvents).
- Electrical Conductivity: Covalent compounds generally do not conduct electricity because they do not have free-moving charged particles like ions. However, some exceptions exist, such as graphite, which exhibits conductivity due to delocalized electrons.
Advanced Concepts and Applications
Our understanding of covalent bonding extends beyond simple diatomic molecules and small organic compounds. Advanced concepts such as resonance structures, molecular orbital theory, and valence bond theory provide a more nuanced picture of bonding in complex molecules and materials.
Resonance Structures
In some molecules, the electron distribution cannot be adequately represented by a single Lewis structure. Resonance structures are used to depict these molecules, showing multiple possible arrangements of electrons that contribute to the overall structure. Benzene (C₆H₆) is a classic example, where the delocalized pi electrons are distributed equally across the ring, leading to resonance stabilization.
Molecular Orbital Theory and Valence Bond Theory
Molecular orbital theory provides a more sophisticated understanding of covalent bonding by considering the combination of atomic orbitals to form molecular orbitals. This theory explains phenomena such as bond order and the magnetic properties of molecules. Valence bond theory, on the other hand, focuses on the overlap of atomic orbitals to form covalent bonds, emphasizing the spatial arrangement of atoms in molecules. Both theories offer valuable insights into the nature of chemical bonding, often complementing each other.
Conclusion
Covalent bonding, the sharing of electron pairs between atoms, is a fundamental concept in chemistry. Understanding the factors influencing covalent bond formation, including electronegativity, bond order, and atomic size, is crucial for predicting and understanding the properties of molecules and materials. From simple diatomic molecules to complex biological macromolecules, the principles of covalent bonding underpin the diversity and complexity of the chemical world. Continued research in this field continues to refine our understanding of the intricate dance of electrons that shapes the world around us. Further exploration into advanced bonding theories provides a deeper appreciation for the subtleties and complexities of this fundamental chemical interaction, driving advancements in materials science, drug design, and numerous other fields.
Latest Posts
Latest Posts
-
What Are The Monomers Of Dna
Mar 17, 2025
-
Does Water Have High Specific Heat
Mar 17, 2025
-
What Are The Three Parameters Of Hypergeometric Pmfs
Mar 17, 2025
-
Difference Between Column And Thin Layer Chromatography
Mar 17, 2025
-
Opening A 6 Membered Ring Mechanism
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Atoms In Molecules Share Pairs Of Electrons When They Make . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
