Atoms Of A Given Element Are Identical
Muz Play
Mar 16, 2025 · 6 min read
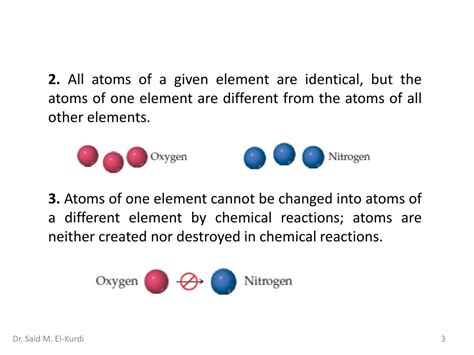
Table of Contents
Atoms of a Given Element are Identical: Exploring the Foundation of Chemistry
The statement "atoms of a given element are identical" is a cornerstone of modern chemistry, a principle that underpins our understanding of matter's composition and behavior. While seemingly simple, this concept, a crucial element of Dalton's atomic theory, requires a nuanced understanding, especially considering the complexities of isotopes and quantum mechanics. This article delves into the intricacies of atomic identity, exploring its historical context, its modern interpretation, and the exceptions that highlight the evolving nature of scientific understanding.
Dalton's Atomic Theory and the Concept of Identical Atoms
John Dalton's atomic theory, proposed in the early 19th century, revolutionized chemistry. One of its key postulates stated that all atoms of a given element are identical in mass and properties. This seemingly straightforward assertion provided a framework for explaining the law of conservation of mass and the law of definite proportions. Dalton envisioned atoms as indivisible, solid spheres, each element possessing its own unique type of atom. This model, while simplistic compared to our current understanding, was a monumental leap forward, laying the foundation for the development of modern chemistry.
The Significance of Identical Atoms in Chemical Reactions
The idea of identical atoms was crucial for understanding chemical reactions. If atoms of an element are identical, then the ratios in which elements combine to form compounds should always be consistent. This principle explains the law of definite proportions, which states that a given compound always contains the same elements in the same proportion by mass. For instance, water (H₂O) always contains two hydrogen atoms for every one oxygen atom. This consistent ratio is a direct consequence of the assumed identity of hydrogen and oxygen atoms.
Isotopes: The Exception that Proves the Rule?
While Dalton's assertion held true as a fundamental principle, the discovery of isotopes introduced a crucial modification. Isotopes are atoms of the same element that have the same number of protons (defining the element) but differ in the number of neutrons. This means that isotopes of the same element have the same atomic number but different mass numbers.
Understanding Isotopic Variation
The presence of isotopes complicates the idea of identical atoms. For example, carbon has three naturally occurring isotopes: carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C). These isotopes all have six protons, but they possess different numbers of neutrons (6, 7, and 8 respectively). This difference in neutron number results in slight variations in their mass and some subtle differences in their chemical behavior, although these differences are generally insignificant for most chemical reactions.
The Impact of Isotopes on Atomic Mass
The existence of isotopes explains why the atomic mass of an element listed on the periodic table is not a whole number. The atomic mass is a weighted average of the masses of all the naturally occurring isotopes of an element, taking into account their relative abundances.
Quantum Mechanics and the Nuances of Atomic Identity
The advent of quantum mechanics further refined our understanding of atoms. The Bohr model, while a significant improvement over Dalton's model, still portrayed electrons orbiting the nucleus in fixed energy levels. Quantum mechanics, however, reveals a more probabilistic description of electron behavior, where electrons occupy orbitals with varying probabilities.
Electron Configuration and Chemical Properties
The electron configuration of an atom, which specifies the arrangement of electrons in its orbitals, largely determines its chemical properties. While isotopes of an element have the same number of protons and electrons, their slightly different nuclear masses can lead to minuscule differences in their electron configurations and consequently, their chemical behavior – an effect known as isotopic fractionation.
Isotopic Effects in Chemical Reactions
Isotopic fractionation, though often small, can be significant in certain situations. For instance, heavier isotopes tend to react more slowly than lighter isotopes due to differences in their vibrational frequencies. This effect is particularly important in fields like geochemistry and paleoclimatology, where isotopic ratios are used to trace the history of geological and biological processes.
Refining the Definition: Chemical Identity vs. Physical Identity
Given the existence of isotopes, we need to refine our understanding of "identical atoms." Atoms of a given element are chemically identical in that they possess the same number of protons and electrons, leading to virtually identical chemical behavior in most reactions. However, they are not physically identical because their masses may differ due to varying numbers of neutrons.
The Importance of Context
The degree to which the differences between isotopes matter depends on the context. In many chemical reactions, the isotopic variations are negligible, and treating all atoms of an element as identical provides a useful simplification. However, in specialized fields like nuclear physics, mass spectrometry, and certain branches of chemistry, the isotopic variations become crucial for understanding specific phenomena.
Beyond Isotopes: Nuclear Isomers and Excited States
The complexities of atomic identity extend beyond isotopes. Nuclear isomers are atoms with the same number of protons and neutrons but exist in different energy states. While they have the same mass number, their different energy levels can affect their nuclear properties and radioactive decay patterns.
Additionally, atoms can exist in excited states, where one or more electrons occupy higher energy levels than in their ground state. These excited states can alter the atom’s reactivity and spectroscopic properties temporarily before returning to the ground state.
Applications and Implications
The principle of chemically identical atoms, even with the caveat of isotopes, underlies many crucial applications:
- Nuclear Medicine: Isotopes like ¹³¹I and ¹⁸F are used in medical imaging and therapy, exploiting the distinct properties of different isotopes of the same element.
- Radiocarbon Dating: The ratio of ¹⁴C to ¹²C in organic materials is used to determine their age, relying on the decay properties of ¹⁴C.
- Geochemistry and Paleoclimatology: Isotopic ratios in rocks and ice cores provide insights into past climates and geological processes.
- Mass Spectrometry: This powerful analytical technique differentiates isotopes based on their mass-to-charge ratio, allowing for precise isotopic analysis.
Conclusion: A Dynamic and Evolving Understanding
The statement "atoms of a given element are identical" is a powerful simplification that remains a cornerstone of chemical understanding. While the discovery of isotopes and the intricacies of quantum mechanics have nuanced this concept, the underlying principle of consistent chemical behavior based on proton number remains valid. The differences between isotopes, though often minor, are significant in specific contexts, enriching our understanding of the natural world and enabling groundbreaking advancements in science and technology. Our understanding of atomic identity is a testament to the dynamic nature of scientific progress, constantly evolving and refining our models to reflect the increasing complexity of the natural world. The journey from Dalton's simple spheres to the complex quantum mechanical description highlights the enduring power of scientific inquiry and the ongoing quest for a deeper comprehension of matter's fundamental constituents.
Latest Posts
Latest Posts
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
-
Evidence Of Light As A Particle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Atoms Of A Given Element Are Identical . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
