Atoms That Gain Or Lose Electrons Are Known As
Muz Play
Mar 25, 2025 · 6 min read
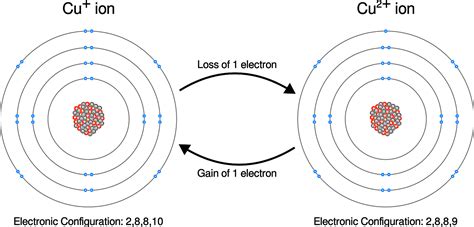
Table of Contents
Atoms That Gain or Lose Electrons Are Known As Ions: A Deep Dive into Ionic Bonds and Their Significance
Atoms are the fundamental building blocks of matter, the tiny particles that make up everything around us. Understanding their behavior, particularly their interactions involving electrons, is crucial to grasping the nature of chemistry and the physical world. This article explores the fascinating phenomenon of atoms gaining or losing electrons, a process that fundamentally alters their properties and leads to the formation of ionic compounds. We will delve into the specifics of ions, their formation, properties, and their significant roles in various scientific fields.
What Happens When Atoms Gain or Lose Electrons?
Atoms, in their neutral state, possess an equal number of protons (positively charged particles in the nucleus) and electrons (negatively charged particles orbiting the nucleus). This balance of positive and negative charges results in a net neutral charge. However, under certain conditions, atoms can either gain or lose electrons, disrupting this balance. This process is crucial for chemical bonding and the formation of various substances.
Atoms that gain electrons become negatively charged. This is because the addition of negatively charged electrons increases the number of negative charges beyond the number of positive protons in the nucleus. These negatively charged atoms are called anions.
Conversely, atoms that lose electrons become positively charged. The loss of negatively charged electrons leaves an excess of positive charges from the protons. These positively charged atoms are called cations.
The process of gaining or losing electrons is often driven by the atom's desire to achieve a stable electron configuration. Many atoms are more stable when they have a full outermost electron shell (also known as the valence shell). This stable configuration is often associated with the noble gases, which have completely filled valence shells. Atoms will readily gain or lose electrons to achieve this state of greater stability.
The Formation of Ions: A Closer Look
The formation of ions is governed by several factors, primarily the atom's electronegativity and its position in the periodic table.
Electronegativity: This property reflects an atom's ability to attract electrons towards itself in a chemical bond. Atoms with high electronegativity tend to gain electrons, forming anions, while atoms with low electronegativity tend to lose electrons, forming cations.
Periodic Table Trends: The periodic table provides a systematic arrangement of elements, reflecting their electron configurations and chemical properties. Elements in the same group (vertical column) share similar chemical properties, often due to similar valence electron numbers.
-
Group 1 (Alkali Metals): These elements readily lose one electron to achieve a stable octet, forming +1 cations (e.g., Na+, K+).
-
Group 2 (Alkaline Earth Metals): These elements readily lose two electrons to achieve a stable octet, forming +2 cations (e.g., Mg2+, Ca2+).
-
Group 17 (Halogens): These elements readily gain one electron to achieve a stable octet, forming -1 anions (e.g., Cl-, Br-).
-
Group 16 (Chalcogens): These elements readily gain two electrons to achieve a stable octet, forming -2 anions (e.g., O2-, S2-).
-
Transition Metals: These elements can lose varying numbers of electrons, leading to the formation of cations with multiple charges (e.g., Fe2+, Fe3+, Cu+, Cu2+).
Ionic Bonds: The Glue That Holds Ions Together
The electrostatic attraction between oppositely charged ions forms an ionic bond. This bond is a strong electrostatic force that holds the cations and anions together in a stable, three-dimensional arrangement known as a crystal lattice. The formation of an ionic bond involves the complete transfer of electrons from one atom to another, resulting in a significant difference in electronegativity between the participating atoms.
Properties of Ionic Compounds
Ionic compounds, formed through ionic bonds, exhibit several characteristic properties:
-
High melting and boiling points: The strong electrostatic forces between ions require significant energy to overcome, leading to high melting and boiling points.
-
Crystalline structure: Ions arrange themselves in a highly ordered, three-dimensional lattice structure, giving ionic compounds their crystalline nature.
-
Solubility in polar solvents: Ionic compounds often dissolve readily in polar solvents like water because the polar water molecules can effectively surround and solvate the ions, reducing the electrostatic attractions between them.
-
Electrical conductivity: Ionic compounds conduct electricity when molten or dissolved in water because the free-moving ions can carry an electric current. In their solid state, the ions are fixed in the crystal lattice and cannot move freely.
-
Brittleness: Ionic crystals are often brittle because a slight shift in the lattice can cause like-charged ions to come into contact, leading to repulsion and fracture.
The Significance of Ions in Biology, Chemistry, and Beyond
Ions play a crucial role in various scientific fields and are essential components of many biological and chemical processes.
Biological Significance:
-
Electrolyte Balance: Ions such as Na+, K+, Ca2+, and Cl- are essential electrolytes in biological systems. They maintain the proper fluid balance, nerve impulse transmission, muscle contractions, and various other physiological processes. Disruptions in electrolyte balance can have serious health consequences.
-
Enzyme Function: Many enzymes require specific ions as cofactors or coenzymes to function correctly. These ions play a critical role in catalyzing biochemical reactions.
-
Bone Structure: Calcium ions (Ca2+) are essential components of bones and teeth, providing structural strength and support.
Chemical Significance:
-
Chemical Reactions: Ions participate in numerous chemical reactions, acting as reactants, intermediates, or products. Understanding the behavior of ions is essential for predicting and controlling chemical reactions.
-
Material Science: Ionic compounds are used extensively in the production of various materials, including ceramics, glasses, and electrolytes for batteries. The properties of these materials are directly related to the nature of the ionic bonds and the crystal structure.
Beyond Biology and Chemistry:
The applications of ions extend beyond biology and chemistry, encompassing various fields such as:
-
Environmental Science: Understanding the ionic composition of water and soil is crucial for assessing water quality and managing environmental pollution.
-
Geochemistry: Ions play a critical role in geological processes, influencing the formation and weathering of rocks and minerals.
-
Medicine: Ions are essential for various medical applications, including electrolyte replacement therapy, drug delivery, and diagnostic imaging.
Conclusion: The Unseen Power of Ions
Atoms that gain or lose electrons, known as ions, are fundamental to understanding the world around us. Their formation, properties, and interactions drive countless chemical and biological processes. From the intricate workings of biological systems to the manufacture of technologically advanced materials, the significance of ions is undeniable. Understanding the concept of ionic bonding and the behavior of ions is not just a cornerstone of chemistry; it's essential for advancements in numerous scientific and technological fields. The study of ions continues to reveal new insights into the fundamental processes shaping our universe, emphasizing their enduring importance and significance across disciplines.
Latest Posts
Latest Posts
-
What Is Pathophysiology Of A Disease
Mar 26, 2025
-
Chi Square Calculator For Goodness Of Fit
Mar 26, 2025
-
Advertising Goals Listed In An Advertising Plan Must Be
Mar 26, 2025
-
What Do Hypotheses Theories And Laws Have In Common
Mar 26, 2025
-
What Are The Products Of Neutralization
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Atoms That Gain Or Lose Electrons Are Known As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
