Balanced Equation Of Decomposition Of Hydrogen Peroxide
Muz Play
May 11, 2025 · 6 min read
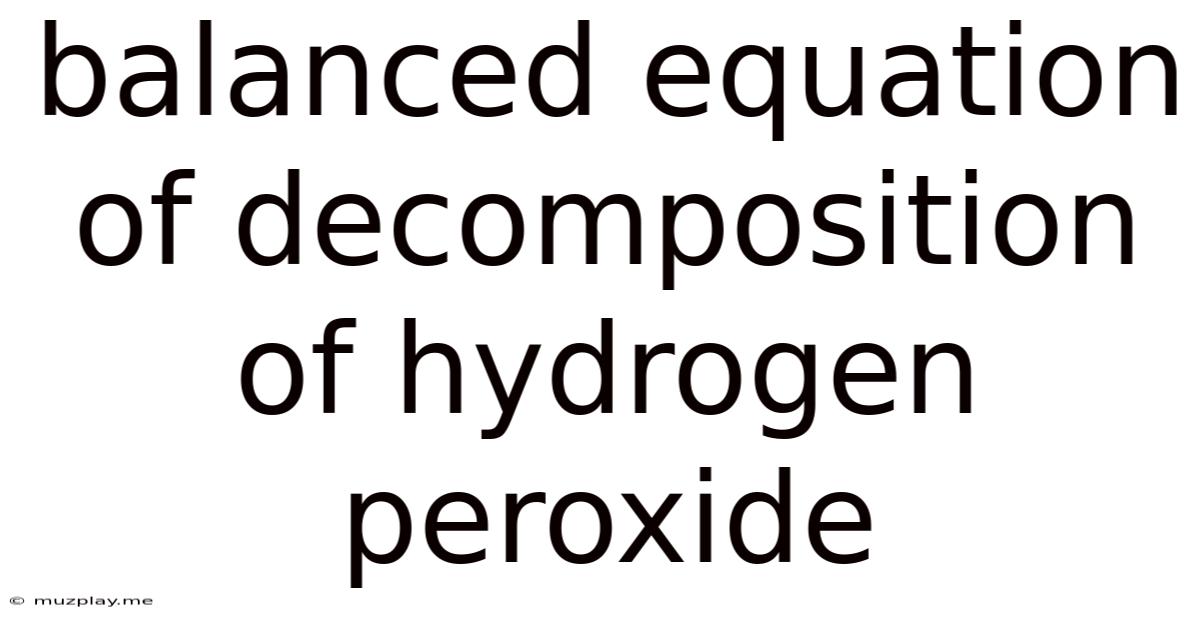
Table of Contents
The Balanced Equation of Hydrogen Peroxide Decomposition: A Deep Dive
Hydrogen peroxide (H₂O₂) is a common chemical compound with a wide range of applications, from disinfectants to rocket fuel. Understanding its decomposition is crucial in various scientific fields. This article delves into the balanced equation for the decomposition of hydrogen peroxide, exploring the reaction mechanisms, factors influencing the rate of decomposition, and its practical implications.
The Decomposition Reaction: A Breakdown
Hydrogen peroxide spontaneously decomposes into water (H₂O) and oxygen gas (O₂). This decomposition is an exothermic reaction, meaning it releases heat. The unbalanced equation for this reaction is:
H₂O₂ → H₂O + O₂
This equation, however, isn't balanced. A balanced chemical equation ensures that the number of atoms of each element is the same on both the reactant (left-hand side) and product (right-hand side) sides of the equation. This adherence to the law of conservation of mass is fundamental in chemistry.
To balance the equation, we need to adjust the coefficients. The balanced equation for the decomposition of hydrogen peroxide is:
2H₂O₂ → 2H₂O + O₂
This balanced equation shows that two molecules of hydrogen peroxide decompose to form two molecules of water and one molecule of oxygen gas. Notice that the number of hydrogen atoms (4) and oxygen atoms (4) is equal on both sides of the equation.
Understanding the Reaction Mechanism
The decomposition of hydrogen peroxide isn't a simple one-step process. It involves a complex series of reactions, often involving free radicals. A common mechanism involves the following steps:
-
Initiation: The decomposition is often initiated by the presence of a catalyst or by heat. This step involves the homolytic cleavage of the peroxide bond (O-O), generating two hydroxyl radicals (.OH).
H₂O₂ → 2•OH
-
Propagation: The hydroxyl radicals react with more hydrogen peroxide molecules, producing water and more hydroxyl radicals in a chain reaction.
•OH + H₂O₂ → H₂O + •OOH
•OOH + H₂O₂ → H₂O + •OH + O₂
-
Termination: The chain reaction eventually terminates when two radicals combine, forming stable molecules. Examples include:
•OH + •OH → H₂O₂
•OH + •OOH → H₂O + O₂
The exact mechanism can vary depending on factors like the presence of catalysts and the reaction conditions.
Factors Affecting the Rate of Decomposition
Several factors influence the rate at which hydrogen peroxide decomposes:
1. Catalysts: Speeding Up the Reaction
Catalysts significantly accelerate the decomposition reaction without being consumed themselves. Common catalysts include:
-
Transition metal ions: Ions of metals like manganese (Mn²⁺), iron (Fe²⁺, Fe³⁺), and copper (Cu²⁺) are effective catalysts. These ions participate in redox reactions within the decomposition mechanism, facilitating the formation of free radicals and speeding up the overall process. Their presence dramatically lowers the activation energy required for the reaction to proceed.
-
Enzymes: Certain enzymes, like catalase, catalyze the decomposition of hydrogen peroxide. Catalase is found in many living organisms, playing a crucial role in protecting cells from the harmful effects of hydrogen peroxide. Catalase efficiently converts hydrogen peroxide into water and oxygen, preventing cellular damage.
-
Other substances: Some other substances, including certain metal oxides and finely divided metals, can also act as catalysts.
The specific choice of catalyst often dictates the reaction rate and selectivity.
2. Temperature: The Heat Factor
The rate of decomposition increases with increasing temperature. Higher temperatures provide the molecules with more kinetic energy, increasing the frequency and effectiveness of collisions, leading to faster decomposition. This is in line with the Arrhenius equation, which describes the relationship between reaction rate and temperature.
3. Concentration: More Reactant, Faster Reaction
Higher concentrations of hydrogen peroxide lead to faster decomposition rates. This is because a higher concentration means more reactant molecules are available for reaction, increasing the likelihood of successful collisions and the formation of products.
4. pH: Acidity and Basicity's Influence
The pH of the solution also affects the decomposition rate. In general, decomposition is faster in acidic or alkaline solutions compared to neutral solutions. The effect of pH is often linked to the activity of catalysts and the stability of the intermediate species involved in the reaction mechanism.
5. Light: Photodecomposition
Exposure to light, particularly ultraviolet (UV) light, can also trigger the decomposition of hydrogen peroxide. UV light possesses sufficient energy to initiate the homolytic cleavage of the peroxide bond, generating free radicals and initiating the decomposition process. This is known as photodecomposition.
Practical Implications of Hydrogen Peroxide Decomposition
Understanding the balanced equation and the factors influencing the decomposition of hydrogen peroxide is critical in various applications:
1. Medical Applications: Disinfection and Wound Healing
Hydrogen peroxide's decomposition is central to its use as an antiseptic and disinfectant. The release of oxygen gas helps to kill bacteria and other microorganisms by disrupting their metabolic processes. The mild oxidizing properties of hydrogen peroxide, combined with the oxygen evolution, make it suitable for cleaning wounds and sterilizing surfaces. However, its use needs careful control, as excessive concentrations can damage healthy tissues.
2. Industrial Applications: Bleaching and Chemical Synthesis
Hydrogen peroxide is a powerful bleaching agent, widely used in the textile, paper, and pulp industries. Its decomposition releases oxygen, which effectively bleaches the materials. The controlled decomposition is crucial in these processes to achieve the desired degree of bleaching without damaging the substrate. It's also a valuable reagent in various chemical syntheses, providing a clean source of oxygen.
3. Environmental Applications: Wastewater Treatment
Hydrogen peroxide is used in advanced oxidation processes (AOPs) for wastewater treatment. AOPs exploit the strong oxidizing power of radicals generated during hydrogen peroxide decomposition to remove organic pollutants from water. The decomposition is carefully controlled to ensure the efficient breakdown of pollutants while minimizing the formation of harmful byproducts.
4. Rocket Propulsion: A Powerful Oxidizer
Hydrogen peroxide has been used as a propellant in rocket propulsion systems. Its decomposition releases a large volume of hot gas, providing thrust for the rocket. Concentrated hydrogen peroxide, sometimes stabilized with additives, is used in various monopropellant and bipropellant systems. The controlled decomposition is a critical aspect of these propulsion systems.
Conclusion: A Versatile Compound with Complex Behavior
The balanced equation for the decomposition of hydrogen peroxide, 2H₂O₂ → 2H₂O + O₂, is a seemingly simple representation of a complex chemical process. This reaction is influenced by a variety of factors, including temperature, concentration, pH, catalysts, and light exposure. Understanding these factors is critical for harnessing the versatile properties of hydrogen peroxide across numerous scientific and industrial applications. From its role as an antiseptic in medicine to its use as a powerful oxidizer in rocket propulsion, hydrogen peroxide's behavior is intrinsically linked to its decomposition reaction, making this balanced equation a cornerstone of its practical applications. Further research continues to explore new and innovative applications of this ubiquitous compound, further highlighting the importance of understanding its chemistry.
Latest Posts
Latest Posts
-
How Is Protein Synthesis Different In Prokaryotes And Eukaryotes
May 12, 2025
-
Biomes With Higher Temperatures And Less Precipitation Tend To Have
May 12, 2025
-
Which Organelle Is Responsible For Making Ribosomes
May 12, 2025
-
What Does Quantized Mean In Chemistry
May 12, 2025
-
Compare And Contrast Evaporation And Condensation
May 12, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation Of Decomposition Of Hydrogen Peroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.