Boiling Point And Freezing Point Of Water
Muz Play
Mar 24, 2025 · 6 min read
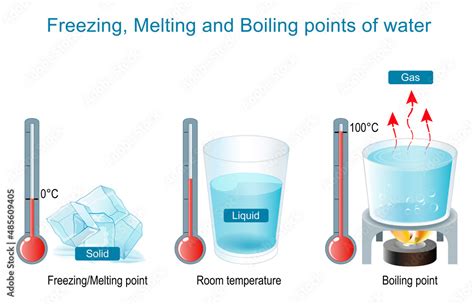
Table of Contents
Boiling Point and Freezing Point of Water: A Deep Dive
Water, the elixir of life, exhibits fascinating properties that are crucial for the existence and sustenance of life on Earth. Among these properties, its boiling point and freezing point stand out as fundamental characteristics influencing numerous natural phenomena and technological processes. This comprehensive exploration delves deep into the science behind these critical points, examining the factors that influence them and their significant implications across various disciplines.
Understanding Boiling Point
The boiling point of a liquid is the temperature at which its vapor pressure equals the external pressure surrounding the liquid. At this point, the liquid transitions into a gaseous state, forming bubbles that rise to the surface. For water, under standard atmospheric pressure (1 atmosphere or 101.325 kPa), the boiling point is 100 degrees Celsius (212 degrees Fahrenheit).
Factors Affecting Boiling Point
Several factors can influence the boiling point of water:
-
Pressure: This is arguably the most significant factor. Higher pressure increases the boiling point because more energy is needed to overcome the increased external force. Conversely, lower pressure lowers the boiling point. This is why water boils at a lower temperature at high altitudes where atmospheric pressure is reduced. Pressure cookers utilize this principle, increasing pressure to raise the boiling point and cook food faster.
-
Impurities: Dissolved substances in water can also affect its boiling point. Generally, the presence of impurities leads to an elevation in the boiling point, a phenomenon known as boiling point elevation. This effect is directly proportional to the concentration of dissolved solute particles.
-
Isotopes: The isotopic composition of water can slightly influence its boiling point. Water molecules containing heavier isotopes of hydrogen (deuterium) or oxygen have slightly higher boiling points than "normal" water. However, this effect is relatively small compared to the influence of pressure and impurities.
Understanding Freezing Point
The freezing point of water, conversely, is the temperature at which it transitions from a liquid state to a solid state (ice). Under standard atmospheric pressure, this point is precisely 0 degrees Celsius (32 degrees Fahrenheit). This seemingly simple number belies a complex interplay of molecular forces and crystal structures.
Factors Affecting Freezing Point
Similar to the boiling point, several factors can alter the freezing point of water:
-
Pressure: While pressure affects both boiling and freezing points, its effect on the freezing point of water is unique. Unlike most substances, increasing pressure on water lowers its freezing point. This is because ice is less dense than liquid water, and applying pressure favors the denser liquid phase. This unusual behavior is crucial for phenomena like ice skating, where the pressure of the skates melts a thin layer of ice, facilitating glide.
-
Impurities: The presence of dissolved substances also affects the freezing point of water, resulting in freezing point depression. This means that adding solutes to water lowers its freezing point. The extent of depression depends on the concentration of the solute. This is the principle behind using antifreeze in car radiators, preventing water from freezing in cold climates.
-
Surface Effects: The freezing point of water can be influenced by surface interactions. For example, water in a narrow capillary tube might freeze at a slightly lower temperature than bulk water due to the influence of the surface.
The Anomalous Behavior of Water
Water's properties, particularly its boiling and freezing points, are considered anomalous compared to other similar molecules. This anomalous behavior arises from the unique hydrogen bonding between water molecules.
Hydrogen Bonding: The Key
Hydrogen bonding is a strong intermolecular force that occurs between a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen in water) and another electronegative atom in a different molecule. This creates a strong attraction between water molecules, leading to several unusual properties:
-
High Boiling Point: Compared to other hydrides in its group (e.g., hydrogen sulfide), water has an unexpectedly high boiling point due to the strong hydrogen bonding. These bonds require more energy to break, hence the higher boiling point.
-
High Heat Capacity: Water has a remarkably high heat capacity, meaning it can absorb a significant amount of heat without a large temperature increase. This property is crucial for regulating Earth's temperature and maintaining stable climates. This is also directly related to the extensive hydrogen bonding network in liquid water.
-
Density Anomaly: Water's density is highest at 4 degrees Celsius. As it cools further towards 0 degrees Celsius, its density decreases. This explains why ice floats on water, a phenomenon with profound ecological implications. This is due to the specific crystalline structure of ice, which maximizes hydrogen bonds but results in a less dense arrangement of molecules than in liquid water.
Implications of Boiling and Freezing Points
The boiling and freezing points of water have profound implications across diverse fields:
-
Biology: Water's unique properties are essential for life as we know it. Its high boiling point allows for liquid water to exist at temperatures suitable for biological processes. The density anomaly ensures that aquatic life can survive even during winter. The high heat capacity of water moderates temperature fluctuations, preventing drastic changes that could harm organisms.
-
Chemistry: Understanding the boiling and freezing points of water is crucial in various chemical processes, including distillation, crystallization, and solution preparation. The use of freezing point depression and boiling point elevation is essential in determining the molar mass of unknown substances.
-
Engineering: Water's boiling and freezing points have significant implications in engineering applications, such as designing cooling systems, heating systems, and various industrial processes. The effects of pressure on boiling and freezing are critical in designing equipment and processes in various industries.
-
Meteorology and Climatology: Water's phase transitions are fundamental to weather patterns and climate dynamics. Evaporation, condensation, freezing, and melting of water drive weather systems, influence rainfall patterns, and play a crucial role in global climate regulation.
Conclusion
The boiling point and freezing point of water, seemingly simple numbers, are actually manifestations of intricate molecular interactions and possess profound implications for the natural world and human activities. Understanding these fundamental properties is crucial for various scientific, technological, and ecological applications. The anomalous behavior of water, largely attributed to hydrogen bonding, sets it apart from other substances and makes it uniquely suited to supporting life on Earth. Further research into the precise mechanisms governing these properties will undoubtedly lead to even deeper insights into the behavior of this ubiquitous and essential substance. Continued investigation into water's properties continues to reveal new complexities and potential applications, solidifying its importance across a wide range of scientific disciplines. The unique properties of water, governed by its molecular structure and interactions, continue to fascinate and inspire scientists and researchers globally.
Latest Posts
Latest Posts
-
How To Find The Basis Of A Matrix
Mar 25, 2025
-
Atoms That Gain Electrons Are Called
Mar 25, 2025
-
How Many Valence Electrons Are In Iron
Mar 25, 2025
-
Solving Equations With Addition And Subtraction
Mar 25, 2025
-
Graphs Of Sine And Cosine Functions Answer Key
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Boiling Point And Freezing Point Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
