Atoms That Gain Electrons Are Called
Muz Play
Mar 25, 2025 · 5 min read
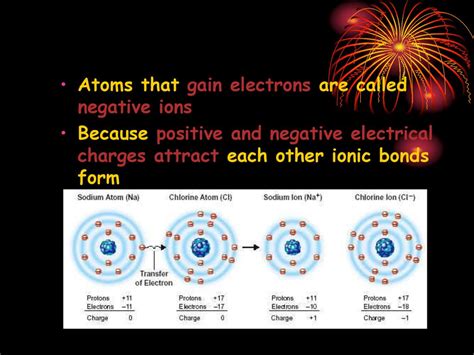
Table of Contents
Atoms That Gain Electrons Are Called: A Deep Dive into Anions and Their Properties
Atoms are the fundamental building blocks of matter, tiny particles that combine to form molecules and everything we see around us. Understanding their behavior, especially concerning electron transfer, is crucial to comprehending chemistry and the physical world. This article delves into the fascinating world of atoms that gain electrons, exploring what they're called, their properties, and their significance in various chemical processes.
What Happens When an Atom Gains Electrons?
When an atom gains one or more electrons, it acquires a negative charge. This is because electrons carry a negative elementary charge, and adding more of them increases the overall negative charge of the atom. This process is fundamentally about achieving a more stable electron configuration. Atoms are most stable when their outermost electron shell (valence shell) is completely filled. By gaining electrons, an atom can achieve this stable configuration, a state of lower energy.
The Octet Rule and Electron Stability
The octet rule is a crucial concept in understanding why atoms gain electrons. This rule states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their valence shell. This configuration mimics the stable electron configuration of noble gases, which are extremely unreactive due to their full valence shells. However, it's important to note that the octet rule isn't absolute; there are exceptions, particularly for elements beyond the second row of the periodic table.
Ionization Energy and Electron Affinity
Two key properties determine an atom's likelihood of gaining electrons:
-
Ionization Energy: This is the energy required to remove an electron from a neutral atom. Atoms with low ionization energies are more likely to lose electrons, while atoms with high ionization energies are less likely to lose electrons and might be more inclined to gain them.
-
Electron Affinity: This is the change in energy when an electron is added to a neutral atom. A high electron affinity indicates that the atom readily accepts an electron and releases energy in the process. Atoms with high electron affinities are more likely to gain electrons.
Atoms That Gain Electrons Are Called Anions
Atoms that gain electrons become negatively charged ions, known as anions. The name "anion" comes from the Greek word "ana," meaning "up," and "ion," referring to the electrically charged particle. The negative charge signifies the excess of electrons over protons within the atom.
Naming Anions
Anion names typically end in "-ide." For example:
- Chloride (Cl⁻): A chlorine atom (Cl) gains one electron to become a chloride ion.
- Sulfide (S²⁻): A sulfur atom (S) gains two electrons to become a sulfide ion.
- Oxide (O²⁻): An oxygen atom (O) gains two electrons to become an oxide ion.
- Nitride (N³⁻): A nitrogen atom (N) gains three electrons to become a nitride ion.
The numerical charge indicates the number of electrons gained. For polyatomic anions (anions composed of multiple atoms), the naming conventions are slightly more complex, often involving prefixes and suffixes that indicate the number of oxygen atoms or the oxidation state of the central atom. For example, sulfate (SO₄²⁻) and phosphate (PO₄³⁻).
The Role of Anions in Chemical Reactions
Anions play crucial roles in a wide variety of chemical reactions and processes:
Ionic Bonding
Anions are fundamental to ionic bonding, a type of chemical bond formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). The transfer of electrons from a metal atom to a non-metal atom creates these oppositely charged ions, which are then held together by strong electrostatic forces. Table salt (NaCl), for example, is formed by the ionic bond between sodium cations (Na⁺) and chloride anions (Cl⁻).
Acid-Base Reactions
Anions are also central to many acid-base reactions. When an acid dissolves in water, it donates protons (H⁺) to water molecules, forming hydronium ions (H₃O⁺) and an anion. The strength of the acid is often related to the stability of the resulting anion. For instance, the strong acid hydrochloric acid (HCl) dissociates completely in water to form H₃O⁺ and Cl⁻, while a weak acid like acetic acid (CH₃COOH) only partially dissociates.
Redox Reactions
Anions participate in redox reactions (reduction-oxidation reactions), which involve the transfer of electrons between species. In these reactions, an anion can either act as an oxidizing agent (accepting electrons and becoming reduced) or a reducing agent (donating electrons and becoming oxidized). The specific behavior depends on the anion's properties and the overall reaction conditions.
Biological Systems
Anions are essential components of biological systems. For instance, phosphate anions (PO₄³⁻) are crucial components of DNA and RNA, the molecules that carry genetic information. Chloride anions (Cl⁻) play a vital role in maintaining the electrolyte balance in the human body. Many other anions are essential cofactors for enzyme function.
Properties of Anions
The properties of anions depend on several factors, including:
- Size: Larger anions generally have lower charge density and are less reactive.
- Charge: Highly charged anions exert stronger electrostatic interactions.
- Electronegativity: The electronegativity of the constituent atoms influences the anion's reactivity.
- Polarizability: The ability of the electron cloud to be distorted affects the anion's interactions with other species.
Examples of Anions and Their Applications
Here are some examples of common anions and their applications:
- Chloride (Cl⁻): Used in table salt, disinfectants, and PVC production.
- Sulfate (SO₄²⁻): Used in fertilizers, detergents, and as a drying agent.
- Nitrate (NO₃⁻): Used in fertilizers and explosives.
- Phosphate (PO₄³⁻): Used in fertilizers, detergents, and as a buffer in biological systems.
- Carbonate (CO₃²⁻): Used in antacids, construction materials, and as a source of carbon dioxide.
- Hydroxide (OH⁻): Used in cleaning products and as a base in chemical reactions.
Conclusion: The Significance of Anions in Chemistry and Beyond
Atoms that gain electrons are called anions, negatively charged ions that play a crucial role in a vast array of chemical processes and biological systems. Their properties, determined by their size, charge, and electronegativity, influence their reactivity and interactions with other species. From the formation of ionic compounds to their involvement in acid-base and redox reactions, anions are indispensable components of the chemical world. Understanding their behavior is essential for comprehending the fundamental principles of chemistry and the workings of the natural world around us. Further research into anion properties and their roles in complex systems continues to reveal the intricate and fascinating nature of these essential chemical building blocks.
Latest Posts
Latest Posts
-
How Do You Calculate The Heat Capacity Of A Calorimeter
Mar 26, 2025
-
Mendels Dihybrid Crosses Supported The Independent Hypothesis
Mar 26, 2025
-
Is Kinetic Energy Conserved In An Elastic Collision
Mar 26, 2025
-
Surplus And Shortage On A Graph
Mar 26, 2025
-
Indicate Whether Or Not The Following Molecules Are Chiral
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Atoms That Gain Electrons Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
