Boiling Water Is A Chemical Change
Muz Play
Mar 31, 2025 · 5 min read
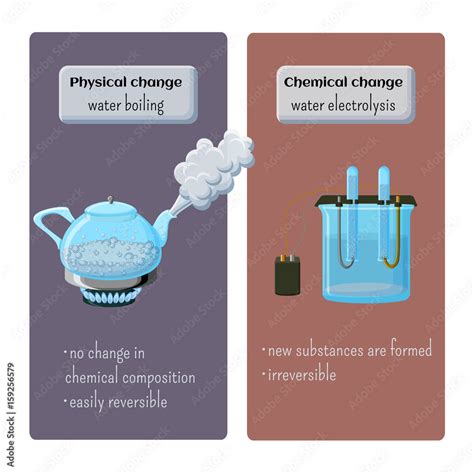
Table of Contents
Is Boiling Water a Chemical Change? A Deep Dive into the Science
The question, "Is boiling water a chemical change?" is a surprisingly complex one, often sparking debate amongst students and enthusiasts alike. While it might seem like a simple physical change—water just shifts from liquid to gas—a closer examination reveals nuances that blur the lines between physical and chemical transformations. This article will delve deep into the science behind boiling water, exploring the arguments for and against classifying it as a chemical change, considering the subtle shifts in molecular behavior and the broader implications of defining chemical changes.
Understanding the Difference: Physical vs. Chemical Changes
Before we tackle the central question, let's establish a clear understanding of the core difference between physical and chemical changes.
Physical changes alter the form or appearance of a substance but not its chemical composition. Think about cutting paper, melting ice, or dissolving sugar in water. The fundamental chemical structure of the substance remains intact. These changes are often reversible.
Chemical changes, also known as chemical reactions, involve a rearrangement of atoms and molecules, resulting in the formation of new substances with different properties. Burning wood, rusting iron, or cooking an egg are all examples of chemical changes. These changes are usually irreversible.
The Case for Boiling Water as a Physical Change
The dominant view in introductory chemistry classes considers boiling water a physical change. The reasoning is straightforward:
-
No new substance is formed: When water boils, it transitions from liquid water (H₂O) to gaseous water (water vapor or steam), still H₂O. The chemical formula remains unchanged. The molecules themselves aren't fundamentally altered; they simply gain enough kinetic energy to overcome intermolecular forces and escape the liquid phase.
-
Reversible process: Condensation, the reverse process of boiling, readily converts water vapor back into liquid water. This reversibility is a strong indicator of a physical change.
-
No significant change in chemical properties: The fundamental properties of water—its polarity, ability to act as a solvent, etc.—remain consistent whether it's in liquid or gaseous form.
The Subtleties: Arguments for a Chemical Change (or a Phase Transition)
While the physical change argument is compelling, a closer look reveals aspects that suggest a more nuanced perspective. This is where we delve into the realm of phase transitions and subtle molecular interactions.
Dissociation of Water Molecules: A Minor Chemical Component
Water, even in its purest form, undergoes a degree of self-ionization. This means that a tiny fraction of water molecules spontaneously dissociate into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). This equilibrium reaction:
2H₂O ⇌ H₃O⁺ + OH⁻
is affected by temperature. Boiling water increases the kinetic energy of the molecules, slightly shifting this equilibrium and altering the concentration of ions, however minimally. This change, although minuscule, represents a chemical alteration, albeit a very minor one.
Changes in Intermolecular Forces: A Defining Feature of Phase Transitions
The transition from liquid to gas involves a significant change in the intermolecular forces holding water molecules together. In liquid water, hydrogen bonds create a relatively strong network of interactions. As water boils, these bonds are disrupted as molecules gain the energy to overcome these attractive forces. While the individual molecules remain intact, the nature and extent of their interactions undergo a dramatic transformation. This is a key characteristic distinguishing different phases of matter, making boiling a more sophisticated process than a mere change in state. These interactions aren't merely physical, they are governed by chemical principles, like the electronegativity of the oxygen and hydrogen atoms and the resulting dipole moments.
The Role of Energy in the Transition: A Chemical Perspective
The energy required to boil water is not just about overcoming intermolecular forces; it's about supplying the energy necessary to break (albeit temporarily) these forces. From a chemical perspective, this energy input directly affects molecular behavior and equilibrium. The heat energy isn't simply changing the kinetic energy of the molecules in a vacuum, it is directly impacting and momentarily altering the chemical balance within the water system.
Phase Transitions: Bridging Physical and Chemical Perspectives
The term "phase transition" highlights the grey area between strictly physical and chemical changes. Boiling, melting, and sublimation are all phase transitions, representing changes in the physical state of a substance without necessarily implying the formation of entirely new chemical species. However, these transitions inevitably involve alterations in molecular interactions, energy levels, and sometimes even minute chemical equilibria, like in the case of water's self-ionization.
To categorize boiling water solely as a physical change overlooks these underlying chemical aspects. While the overall chemical composition remains unchanged, the process itself involves measurable chemical changes at a molecular level. Therefore, a more accurate description might be that boiling water is a physical process with subtle chemical components.
Conclusion: A Nuanced Understanding
The question of whether boiling water is a chemical change is not a simple "yes" or "no." The traditional perspective of boiling as a purely physical change holds true in a simplified context. However, a more comprehensive understanding reveals subtle yet significant chemical aspects associated with the process. The changes in molecular interactions, the slight shift in the self-ionization equilibrium, and the crucial role of energy input all point to a more complex reality that transcends the simple physical-chemical dichotomy.
Therefore, classifying boiling water solely as a physical change is an oversimplification. A more nuanced approach recognizes it as a physical process with inherent chemical components, a phase transition driven by chemical principles and impacting chemical equilibria, albeit at a minuscule scale in the case of water's self-ionization. This richer understanding acknowledges the intricacies of molecular behavior and the dynamic interplay between physical and chemical forces in everyday phenomena. It highlights the need for careful consideration of multiple factors when categorizing changes in matter, fostering a deeper appreciation for the complexity of the chemical world. The debate isn't about being right or wrong, but rather about progressing to a more accurate and complete scientific understanding.
Latest Posts
Latest Posts
-
The Most Reactive Group In The Periodic Table
Apr 02, 2025
-
How To Write Quadratic Equation From Graph
Apr 02, 2025
-
How To Place A Condom Catheter
Apr 02, 2025
-
How Many Elements Are Gases At Room Temperature
Apr 02, 2025
-
3 Main Ideas Of Cell Theory
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Is A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
