Bronsted Lowry Acid Vs Lewis Acid
Muz Play
Mar 16, 2025 · 5 min read
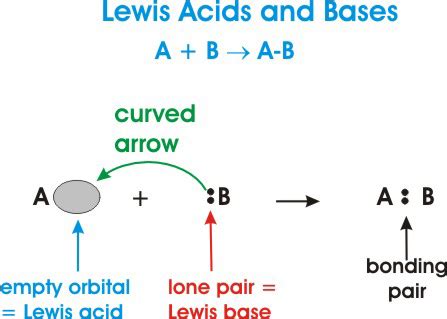
Table of Contents
Brønsted-Lowry Acid vs. Lewis Acid: A Comprehensive Comparison
Understanding the concepts of acids and bases is fundamental to chemistry. While the Brønsted-Lowry and Lewis definitions both describe acids and bases, they differ significantly in their scope and application. This article delves into the nuances of each definition, highlighting their similarities, differences, and providing examples to solidify your understanding.
Brønsted-Lowry Acid-Base Theory: The Proton Transfer Paradigm
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, centers around the transfer of protons (H⁺ ions). According to this theory:
- Brønsted-Lowry Acid: A substance that donates a proton (H⁺) to another substance.
- Brønsted-Lowry Base: A substance that accepts a proton (H⁺) from another substance.
This theory expands upon the simpler Arrhenius definition (which restricts acids and bases to aqueous solutions) by encompassing reactions that don't necessarily involve water. The key here is the proton transfer. A Brønsted-Lowry acid-base reaction always involves a conjugate acid-base pair.
Understanding Conjugate Acid-Base Pairs
When an acid donates a proton, it forms its conjugate base. Similarly, when a base accepts a proton, it forms its conjugate acid. These pairs are related by the difference of a single proton.
Example: Consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
In this reaction:
- HCl is the acid (proton donor).
- H₂O is the base (proton acceptor).
- H₃O⁺ (hydronium ion) is the conjugate acid of H₂O.
- Cl⁻ (chloride ion) is the conjugate base of HCl.
Key features of Brønsted-Lowry theory:
- Focuses on proton transfer: The defining characteristic is the movement of a proton.
- Includes reactions without water: Reactions in non-aqueous solvents are also encompassed.
- Conjugate acid-base pairs: Every acid has a conjugate base, and every base has a conjugate acid.
- Amphoteric substances: Some substances can act as both acids and bases, depending on the reaction conditions (e.g., water).
Lewis Acid-Base Theory: A Broader Perspective
Gilbert N. Lewis proposed a more general definition of acids and bases in 1923, focusing on electron pairs rather than proton transfer. This theory significantly broadens the scope of acid-base chemistry:
- Lewis Acid: A substance that accepts an electron pair. These are often electron-deficient species.
- Lewis Base: A substance that donates an electron pair. These often have lone pairs of electrons.
The Lewis definition encompasses many reactions that are not considered acid-base reactions under the Brønsted-Lowry definition. The key here is the donation and acceptance of an electron pair, forming a coordinate covalent bond.
Distinguishing Lewis Acids and Bases
Many common Brønsted-Lowry acids are also Lewis acids because they can accept electron pairs. However, many substances that are not Brønsted-Lowry acids can be classified as Lewis acids.
Examples:
- BF₃ (Boron trifluoride): BF₃ is a Lewis acid because boron has an empty p-orbital and can accept an electron pair.
- AlCl₃ (Aluminum chloride): Similar to BF₃, AlCl₃ can accept an electron pairs.
- NH₃ (Ammonia): NH₃ is a Lewis base because the nitrogen atom has a lone pair of electrons that it can donate.
Lewis Acid-Base Reactions: Beyond Proton Transfer
Lewis acid-base reactions often involve the formation of a coordinate covalent bond, where both electrons in the bond come from the Lewis base.
Example: The reaction between BF₃ and NH₃:
BF₃ + NH₃ → F₃B-NH₃
In this reaction:
- BF₃ is the Lewis acid (electron pair acceptor).
- NH₃ is the Lewis base (electron pair donor).
- A coordinate covalent bond is formed between boron and nitrogen.
Key features of Lewis theory:
- Focuses on electron pair transfer: The defining characteristic is the donation and acceptance of an electron pair.
- Broader scope than Brønsted-Lowry: Includes reactions not involving protons.
- Explains reactions with no protons: Many reactions involving metal ions and ligands are explained by the Lewis theory.
- Predicts reaction products based on electron configuration: Helps understand the reactivity of various species.
Comparing Brønsted-Lowry and Lewis Acid-Base Theories
| Feature | Brønsted-Lowry Theory | Lewis Theory |
|---|---|---|
| Definition | Proton (H⁺) transfer | Electron pair transfer |
| Acid | Proton donor | Electron pair acceptor |
| Base | Proton acceptor | Electron pair donor |
| Scope | More limited, focuses on proton transfer | Broader, encompasses many more reactions |
| Examples | HCl, H₂SO₄, NH₃ (as a base) | BF₃, AlCl₃, NH₃ (as a base), many metal ions |
| Conjugates | Conjugate acid-base pairs are central | No direct equivalent of conjugate pairs |
Applications and Significance
Both theories are crucial in various fields of chemistry and beyond:
- Inorganic Chemistry: Understanding metal-ligand interactions is heavily reliant on the Lewis acid-base theory. Many coordination complexes are formed through Lewis acid-base interactions.
- Organic Chemistry: The Brønsted-Lowry theory is vital for understanding acid-catalyzed reactions and the behavior of organic acids and bases. The Lewis theory helps explain reactions involving carbocations and carbanions.
- Biochemistry: Numerous biological processes involve acid-base reactions, including enzyme catalysis and the regulation of pH in biological systems. Both theories are crucial here.
- Environmental Science: Acid rain, a significant environmental problem, is explained using the Brønsted-Lowry theory.
- Material Science: The Lewis acid-base theory plays a crucial role in the design and synthesis of new materials.
Conclusion: A Unified Perspective
While the Brønsted-Lowry theory provides a valuable framework for understanding many acid-base reactions, the Lewis theory offers a more comprehensive and versatile approach. The Lewis theory essentially encompasses the Brønsted-Lowry theory as a subset. Understanding both definitions is essential for a complete grasp of acid-base chemistry and its wide-ranging applications in various scientific disciplines. Choosing between the two often depends on the specific context of the reaction being studied and the level of detail required for understanding the underlying mechanism. Both theories are powerful tools for analyzing and predicting chemical behavior. The flexibility and broader scope of the Lewis definition makes it particularly useful when dealing with more complex reactions and chemical systems. Remember that the core concepts of electron pair donation and acceptance provide a more fundamental understanding of chemical bonding and reactivity.
Latest Posts
Latest Posts
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Bronsted Lowry Acid Vs Lewis Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
