Brønsted Lowry Conjugate Acid Base Pair
Muz Play
Mar 28, 2025 · 6 min read
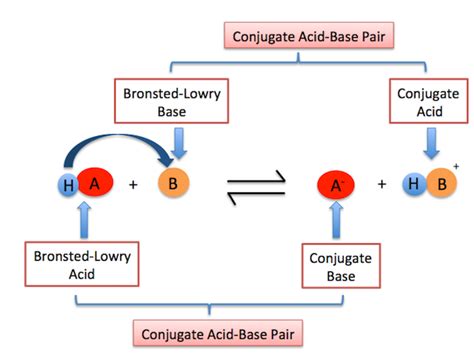
Table of Contents
Brønsted-Lowry Conjugate Acid-Base Pairs: A Deep Dive
Understanding acids and bases is fundamental to chemistry. While several theories define acids and bases (like Arrhenius and Lewis theories), the Brønsted-Lowry theory provides a particularly insightful perspective, focusing on the crucial role of proton transfer. This theory introduces the concept of conjugate acid-base pairs, a cornerstone for comprehending acid-base reactions and equilibria. This comprehensive article will explore this concept in detail, covering definitions, examples, applications, and even some nuanced considerations.
Defining Brønsted-Lowry Acids and Bases
The Brønsted-Lowry theory defines an acid as a proton donor and a base as a proton acceptor. A proton, in this context, refers to a hydrogen ion (H⁺). Unlike the Arrhenius theory which limits acids and bases to aqueous solutions, the Brønsted-Lowry definition expands the scope to encompass a broader range of reactions, including those in non-aqueous solvents or even in the gas phase.
Crucially, acid-base reactions according to this theory always involve the transfer of a proton from the acid to the base. This transfer is the defining characteristic of a Brønsted-Lowry acid-base reaction.
Conjugate Acid-Base Pairs: The Heart of the Matter
This proton transfer doesn't leave the base and the acid unchanged. The acid, after donating its proton, becomes a conjugate base, while the base, after accepting a proton, becomes a conjugate acid. This pair – the original acid and its resulting conjugate base, or the original base and its resulting conjugate acid – is known as a conjugate acid-base pair.
The relationship between a conjugate acid-base pair is characterized by a difference of just one proton (H⁺). The conjugate acid has one more proton than its conjugate base. This seemingly simple difference has significant implications for the reactivity and properties of these species.
Example 1: The Classic Case of HCl and Water
Consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Cl⁻(aq)
In this reaction:
- HCl acts as the acid, donating a proton (H⁺).
- H₂O acts as the base, accepting the proton.
- Cl⁻ is the conjugate base of HCl. It's what remains of HCl after losing a proton.
- H₃O⁺ (hydronium ion) is the conjugate acid of H₂O. It's the species formed when H₂O accepts a proton.
Thus, (HCl, Cl⁻) and (H₂O, H₃O⁺) are two conjugate acid-base pairs in this reaction.
Example 2: Ammonia and Water
Let's look at another common example: the reaction between ammonia (NH₃) and water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Here:
- NH₃ acts as the base, accepting a proton.
- H₂O acts as the acid, donating a proton.
- NH₄⁺ (ammonium ion) is the conjugate acid of NH₃.
- OH⁻ (hydroxide ion) is the conjugate base of H₂O.
Therefore, (NH₃, NH₄⁺) and (H₂O, OH⁻) form two conjugate acid-base pairs.
Strength of Conjugate Acid-Base Pairs: A Reciprocal Relationship
The strength of an acid is directly related to the strength of its conjugate base. Strong acids have weak conjugate bases, and weak acids have strong conjugate bases. This inverse relationship stems from the fact that a strong acid readily donates its proton, leaving behind a conjugate base that has little tendency to accept a proton back. Conversely, a weak acid holds onto its proton tightly, resulting in a conjugate base that readily accepts a proton.
Similarly, strong bases have weak conjugate acids, and weak bases have strong conjugate acids. This reciprocal relationship is crucial for predicting the position of equilibrium in acid-base reactions.
Identifying Conjugate Acid-Base Pairs: A Practical Guide
Identifying conjugate acid-base pairs involves a simple process:
- Identify the proton transfer: Determine which species donates a proton and which species accepts a proton.
- Locate the difference: The conjugate acid will have one more proton than its conjugate base. Look for the species that differ by only a single proton.
- Pair them up: The proton donor and its resulting species form one conjugate pair, and the proton acceptor and its resulting species form the other.
Practice is key! Working through numerous examples will solidify your understanding of this concept.
Applications of Conjugate Acid-Base Pairs
The concept of conjugate acid-base pairs is not merely an academic exercise; it has wide-ranging applications across various fields:
-
Buffer solutions: Buffer solutions are crucial in maintaining a relatively constant pH. They typically consist of a weak acid and its conjugate base (or a weak base and its conjugate acid). The presence of both species allows the buffer to resist changes in pH upon the addition of small amounts of acid or base. Understanding the conjugate acid-base relationship is essential for designing and predicting the effectiveness of buffer solutions.
-
Acid-base titrations: Titration curves, used extensively in analytical chemistry to determine the concentration of an unknown acid or base, are deeply intertwined with the behavior of conjugate acid-base pairs. The equivalence point, where the acid and base completely neutralize each other, is directly related to the properties of the conjugate acid and base formed.
-
Enzyme catalysis: Many enzymes, biological catalysts, utilize acid-base catalysis mechanisms involving proton transfer. Understanding conjugate acid-base pairs is vital in comprehending how these enzymes function at a molecular level. The active sites of these enzymes often contain acidic or basic residues that act as proton donors or acceptors, facilitating reactions within the cell.
-
Organic chemistry reactions: Numerous organic reactions involve proton transfer steps, frequently involving conjugate acid-base pairs. For instance, reactions involving carbonyl compounds, amines, and alcohols often proceed via mechanisms involving protonation and deprotonation. Knowing the acidity and basicity of various functional groups and their resulting conjugate pairs helps in predicting reaction pathways and outcomes.
Nuanced Considerations: Beyond the Basics
While the fundamental concept of conjugate acid-base pairs is relatively straightforward, some nuances deserve attention:
-
Amphoteric substances: Some substances can act as both acids and bases, depending on the reaction context. Water is a classic example; it can act as an acid (donating a proton to NH₃) or a base (accepting a proton from HCl). These substances can participate in forming multiple conjugate pairs simultaneously within a single reaction.
-
Polyprotic acids and bases: These substances can donate or accept multiple protons. Phosphoric acid (H₃PO₄), for example, can donate three protons sequentially, forming a series of conjugate base pairs. Understanding the stepwise dissociation of these acids and bases is crucial in predicting their behavior in solution.
Conclusion: Mastering Conjugate Acid-Base Pairs
The Brønsted-Lowry theory, with its emphasis on proton transfer and the concept of conjugate acid-base pairs, provides a powerful framework for understanding acid-base chemistry. Mastering this concept is crucial for anyone studying chemistry, from introductory courses to advanced research. By understanding the reciprocal relationship between acid strength and conjugate base strength, and by practicing identifying conjugate pairs in various reaction scenarios, you will develop a solid foundation in acid-base chemistry and its numerous applications across diverse scientific disciplines. The insights gained from this understanding pave the way for deeper exploration of topics like equilibrium, kinetics, and the role of acids and bases in complex chemical systems. Remember, consistent practice and attention to the nuances of proton transfer reactions are key to a comprehensive grasp of this essential chemical concept.
Latest Posts
Latest Posts
-
Keratin And Collagen Are Examples Of Which Class Of Proteins
Mar 31, 2025
-
Job Order Costing Vs Process Costing
Mar 31, 2025
-
What Is The Chemical Equation For Aerobic Respiration
Mar 31, 2025
-
Equation Of The Tangent Line Implicit Differentiation
Mar 31, 2025
-
What Is The Secondary Structure Of Dna
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Brønsted Lowry Conjugate Acid Base Pair . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
