C Double Bond C Ir Spectrum
Muz Play
Mar 22, 2025 · 6 min read
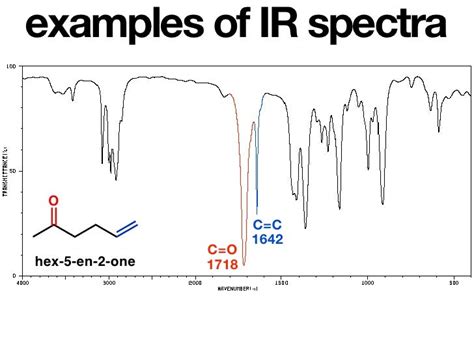
Table of Contents
C=C Double Bond IR Spectrum: A Comprehensive Guide
The infrared (IR) spectrum is a powerful tool used in organic chemistry to identify functional groups within a molecule. One of the most readily identifiable features in an IR spectrum is the presence of a carbon-carbon double bond (C=C). While not as intense as some other functional group absorptions, understanding the nuances of the C=C stretch in IR spectroscopy can provide valuable information about the structure and environment of the alkene. This comprehensive guide delves into the intricacies of the C=C double bond IR spectrum, exploring its characteristic features, influencing factors, and practical applications.
Understanding the C=C Stretching Vibration
The absorption band associated with the C=C stretching vibration arises from the stretching motion of the two carbon atoms involved in the double bond. This vibration is inherently weaker than the stretching vibrations of polar bonds like O-H, N-H, and C=O, due to the relatively non-polar nature of the C=C bond. Consequently, the absorption intensity is generally lower.
Characteristic Frequency Range
The C=C stretching vibration typically appears in the 1680-1620 cm⁻¹ region of the IR spectrum. However, this range is quite broad and can shift depending on several factors which we will explore below. The exact frequency depends on several structural factors, and interpreting the precise frequency often requires considering these alongside other spectral data.
Factors Influencing the C=C Stretching Frequency
Several factors influence the precise frequency of the C=C stretching vibration:
-
Substitution: The degree of substitution significantly affects the absorption frequency. Tetrasubstituted alkenes generally exhibit the lowest frequency, typically around 1620-1640 cm⁻¹, due to the increased mass and electron delocalization. Trisubstituted alkenes absorb slightly higher, usually in the 1640-1650 cm⁻¹ range. Disubstituted alkenes show absorptions around 1660-1680 cm⁻¹. Finally, monosubstituted alkenes typically display absorption at higher frequencies, generally in the 1660-1680 cm⁻¹ range, though this can vary.
-
Conjugation: Conjugation with other π systems, such as carbonyl groups or aromatic rings, significantly lowers the C=C stretching frequency. This is because conjugation delocalizes the electrons over a larger region, weakening the bond and reducing its vibrational frequency. Conjugated systems can exhibit absorption bands as low as 1600 cm⁻¹. The presence of conjugation is often indicated by a shift in the frequency and an increase in the intensity of the absorption band.
-
Ring Strain: Cyclic alkenes with significant ring strain, such as cyclopropenes, exhibit higher C=C stretching frequencies due to the increased bond strength caused by the strain.
-
Hydrogen Bonding: While less pronounced than with other functional groups, hydrogen bonding can weakly influence the C=C stretching frequency. The effect is typically a minor shift to lower frequencies.
-
Inductive Effects: Electronegative substituents on the alkene can induce a slight shift in the C=C stretching frequency due to their effect on the electron density of the double bond.
Identifying the C=C Peak in an IR Spectrum
While the C=C stretch is relatively weak, several characteristics can assist in its identification:
-
Weak Intensity: As mentioned previously, the C=C stretch is typically a weak absorption compared to other functional groups.
-
Sharp Band: The band is usually quite sharp and well-defined, making it relatively easy to distinguish from broader absorptions.
-
Frequency Range: Remember the characteristic range (1680-1620 cm⁻¹), and consider the substituent and structural effects mentioned above.
-
Comparison with other Spectral Data: Combining IR data with other spectroscopic techniques such as NMR and mass spectrometry is crucial for accurate structural elucidation. The C=C stretch should be consistent with the overall structural information gleaned from other analytical methods.
-
Absence of Other Absorptions: In cases where a peak around 1650 cm⁻¹ is observed, confirm there are no strong absorptions in other regions indicative of other functional groups that could overlap (e.g., strong C=O stretch).
-
Solvent Effects: Note that the solvent used in recording the IR spectrum can induce slight shifts in the absorption frequencies. It is important to always note the solvent used for proper comparison and interpretation of data.
Practical Applications and Examples
The information gained from the C=C stretching frequency and intensity is invaluable in the identification and characterization of alkenes. Here are some practical examples:
-
Distinguishing between Alkanes and Alkenes: The presence of a peak in the 1680-1620 cm⁻¹ region is strong evidence for the existence of a C=C double bond, differentiating an alkene from an alkane.
-
Determining the Degree of Unsaturation: While not directly providing the number of double bonds, the presence and number of peaks in this region, coupled with other data, can support information about the degree of unsaturation within the molecule.
-
Identifying Conjugation: A shift to lower frequencies (below 1650 cm⁻¹) strongly suggests the presence of conjugation.
-
Analyzing Polymer Structure: IR spectroscopy is used extensively in polymer chemistry to analyze the structure and composition of polymers containing alkene units. The C=C stretching frequency can help determine the type and degree of unsaturation in the polymer backbone.
-
Monitoring Reaction Progress: The changes in the intensity or frequency of the C=C stretching band can be used to monitor the progress of reactions involving alkenes, such as addition or polymerization reactions.
Advanced Considerations and Limitations
While IR spectroscopy is a powerful technique, it’s important to acknowledge its limitations regarding C=C bond identification:
-
Overlapping Peaks: In complex molecules, the C=C stretching band might overlap with other absorptions, making accurate identification challenging.
-
Weak Intensity: The inherent weakness of the C=C absorption can lead to difficulties in detecting the band in low concentrations or the presence of stronger absorbing functional groups.
-
Ambiguity without Context: Determining the precise structure and substitution pattern solely from the C=C stretch frequency can be ambiguous without additional spectral information. Combining IR data with other techniques is always recommended for complete structural elucidation.
Conclusion
The C=C double bond IR spectrum provides valuable insights into the structural features of alkenes. While the absorption is relatively weak, understanding its characteristic frequency range, the factors that influence it, and the potential for overlapping peaks, provides a robust tool for identification. By combining IR spectroscopy with other analytical techniques, a complete and accurate picture of the molecular structure can be achieved. Careful analysis of the peak's frequency, intensity, and shape, in conjunction with other spectral data, allows chemists to accurately characterize the presence and environment of carbon-carbon double bonds in a wide range of organic molecules. Remember to always carefully consider the complete spectral context and to integrate IR data with other analytical techniques for the most accurate and reliable interpretations.
Latest Posts
Latest Posts
-
3 X 2 Factorial Design Example
Mar 22, 2025
-
What Type Of Solid Is Diamond
Mar 22, 2025
-
Do Electrons Have A Smaller Mass Than Protons And Neutrons
Mar 22, 2025
-
What Is The Building Block Of A Nucleic Acid Called
Mar 22, 2025
-
How To Solve Exact Differential Equations
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about C Double Bond C Ir Spectrum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
