Do Electrons Have A Smaller Mass Than Protons And Neutrons
Muz Play
Mar 22, 2025 · 6 min read
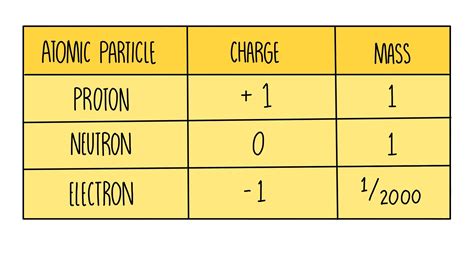
Table of Contents
Do Electrons Have a Smaller Mass Than Protons and Neutrons? A Deep Dive into Subatomic Particles
The question of whether electrons have a smaller mass than protons and neutrons is fundamental to our understanding of atomic structure and the universe itself. The answer is a resounding yes, and understanding why this is the case requires delving into the fascinating world of subatomic particles and their properties. This article will explore the relative masses of these particles, examining the experimental evidence, the implications for atomic behavior, and the underlying theoretical frameworks that explain these differences.
The Relative Masses of Subatomic Particles
Electrons, protons, and neutrons are the three primary constituents of atoms. However, their masses differ significantly. To understand these differences, we typically express the masses relative to the atomic mass unit (amu), which is defined as one-twelfth the mass of a carbon-12 atom. While using precise mass values in kilograms is possible, using amu simplifies comparisons and calculations in the context of atomic physics.
Electron Mass: A Featherweight Champion
The electron's mass is incredibly small, approximately 0.00054858 amu. This translates to roughly 9.109 x 10<sup>-31</sup> kg. Its minuscule mass is a key factor determining its behavior in atoms and its participation in various physical phenomena.
Proton Mass: The Heavyweight Contender
Protons, on the other hand, are much more massive. Their mass is approximately 1.007276 amu, or about 1.673 x 10<sup>-27</sup> kg. This represents a substantial difference compared to the electron, being nearly 1836 times more massive.
Neutron Mass: A Close Cousin to the Proton
Neutrons have a mass very similar to protons, weighing in at approximately 1.008665 amu or 1.675 x 10<sup>-27</sup> kg. The neutron is slightly heavier than the proton, but the difference is relatively small in comparison to the disparity between these particles and the electron.
Experimental Evidence and Measurement Techniques
The determination of the masses of these subatomic particles has been a significant achievement in scientific history, relying on a combination of experimental techniques. These include:
Mass Spectrometry: Weighing the Atoms
Mass spectrometry is a powerful tool used to determine the mass-to-charge ratio of ions. By accelerating ions in a magnetic field, scientists can separate them based on their mass-to-charge ratio, enabling the precise measurement of the masses of isotopes and consequently, the constituents of atoms. Different variations of mass spectrometry exist, each offering improved precision and sensitivity.
Particle Accelerators: Unveiling the Subatomic World
Particle accelerators, such as cyclotrons and synchrotrons, play a crucial role in high-energy physics experiments. By accelerating charged particles to incredibly high speeds, these machines allow physicists to study the interactions between subatomic particles, providing insights into their properties, including their masses. The analysis of collision products helps determine the masses of the participating particles with astonishing accuracy.
Other Techniques: Precision Measurements and Refinements
Further techniques, like precision measurements of atomic spectra and the analysis of radioactive decay processes, have contributed to refining the values of these masses. Continuous advancements in experimental techniques have led to increasingly precise measurements, pushing the limits of our understanding of the subatomic realm.
The Implications of Mass Differences for Atomic Behavior
The significant difference in mass between electrons and protons/neutrons has profound implications for atomic structure and behavior:
Electron Orbitals and Atomic Size:
The relatively small mass of the electron allows it to occupy a much larger volume of space around the nucleus compared to the protons and neutrons. This is crucial for the formation of electron orbitals and ultimately determines the size of an atom. The significantly heavier protons and neutrons remain tightly bound in the nucleus, forming its core.
Chemical Bonding:
The mass difference is indirectly involved in the formation of chemical bonds. Electrons are responsible for chemical interactions due to their mobility and capacity to participate in electrostatic interactions. The heavier protons and neutrons, being concentrated in the nucleus, play a less direct role in chemical bonding, primarily determining the overall mass of the atom and influencing its nuclear stability.
Nuclear Reactions and Stability:
The masses of protons and neutrons are critically important in understanding nuclear reactions and the stability of atomic nuclei. The mass difference between the nucleus and the sum of the masses of its constituent protons and neutrons accounts for the binding energy that holds the nucleus together.
Theoretical Frameworks and Explanations
The differences in the masses of electrons, protons, and neutrons are explained by the Standard Model of particle physics. This model postulates that protons and neutrons are not fundamental particles but are composed of more elementary constituents called quarks.
Quarks: The Building Blocks of Protons and Neutrons
Protons and neutrons each consist of three quarks:
- Proton: Two up quarks and one down quark (uud)
- Neutron: One up quark and two down quarks (udd)
These quarks have their own masses and interact through the strong nuclear force, which is responsible for binding them together within protons and neutrons. The overall mass of a proton or neutron is significantly greater than the sum of the masses of its constituent quarks due to the contribution of the strong force's binding energy, a concept explained by Einstein's famous equation, E=mc².
Leptons: The Electron's Family
Electrons belong to a category of particles called leptons, which are fundamental particles—meaning they are not made up of smaller constituents as far as we currently know. The electron's mass is an intrinsic property, determined by its interaction with the Higgs field, a fundamental field permeating all of space. The interaction with the Higgs field is responsible for giving particles mass. The electron interacts relatively weakly with the Higgs field, resulting in its small mass.
Conclusion: A Tale of Two Masses (or Three!)
The fact that electrons have a significantly smaller mass than protons and neutrons is a cornerstone of atomic physics. This mass difference is crucial for understanding atomic structure, chemical bonding, nuclear reactions, and the behavior of matter in general. The disparity in mass is a consequence of the different fundamental constituents of these particles and their interactions with fundamental forces. The electron, a fundamental lepton, interacts weakly with the Higgs field, while protons and neutrons are composites of quarks bound together by the strong force, contributing significantly to their greater mass. The experimental techniques used to measure these masses have been refined over time, resulting in increasingly accurate values that underpin our current understanding of the universe at the subatomic level. Ongoing research continues to explore the intricacies of particle physics, promising further revelations about the fundamental nature of matter and its constituents.
Latest Posts
Latest Posts
-
4 Most Common Elements In Living Organisms
Mar 23, 2025
-
How To Find The Change In Enthalpy
Mar 23, 2025
-
Most Reactive Elements On The Periodic Table
Mar 23, 2025
-
How To Convert Atoms To Grams
Mar 23, 2025
-
Male And Female Pelvis Differences Table
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Do Electrons Have A Smaller Mass Than Protons And Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
