How To Find The Change In Enthalpy
Muz Play
Mar 23, 2025 · 6 min read
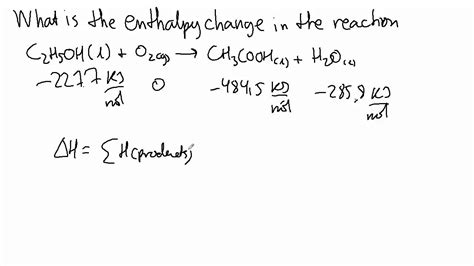
Table of Contents
How to Find the Change in Enthalpy: A Comprehensive Guide
Enthalpy, denoted as H, is a thermodynamic property representing the total heat content of a system at constant pressure. The change in enthalpy (ΔH) signifies the heat absorbed or released during a process at constant pressure. Understanding how to calculate ΔH is crucial in various fields, including chemistry, physics, and engineering. This comprehensive guide will delve into different methods for determining the change in enthalpy, covering various scenarios and providing practical examples.
Understanding Enthalpy Change (ΔH)
Before exploring the methods, let's solidify our understanding of ΔH. A positive ΔH indicates an endothermic process, where the system absorbs heat from its surroundings. Conversely, a negative ΔH represents an exothermic process, where the system releases heat to its surroundings. The magnitude of ΔH reflects the amount of heat exchanged.
Several factors influence ΔH, including:
- Type of reaction: Different reactions have different enthalpy changes. Combustion reactions, for example, are typically highly exothermic.
- State of reactants and products: The physical states (solid, liquid, gas) of the reactants and products significantly impact ΔH.
- Temperature and pressure: While ΔH is often reported at standard conditions (298 K and 1 atm), changes in temperature and pressure can affect its value.
- Stoichiometry of the reaction: The balanced chemical equation provides the molar ratios, crucial for calculating ΔH for specific amounts of reactants or products.
Methods for Determining ΔH
Several methods exist for determining the change in enthalpy, each with its own applicability and limitations. Let's explore some of the most common:
1. Using Standard Enthalpies of Formation (ΔHf°)
This is arguably the most common method for calculating ΔH. The standard enthalpy of formation (ΔHf°) is the change in enthalpy when one mole of a compound is formed from its constituent elements in their standard states (usually at 298 K and 1 atm). These values are tabulated for many compounds and elements.
The formula for calculating ΔH using standard enthalpies of formation is:
ΔH°<sub>rxn</sub> = Σ [ΔHf°(products)] - Σ [ΔHf°(reactants)]
Where:
- ΔH°<sub>rxn</sub> is the standard enthalpy change of the reaction.
- Σ [ΔHf°(products)] is the sum of the standard enthalpies of formation of the products.
- Σ [ΔHf°(reactants)] is the sum of the standard enthalpies of formation of the reactants.
Example:
Consider the combustion of methane: CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
To calculate ΔH°<sub>rxn</sub>, we need the standard enthalpies of formation for each compound. These values are typically found in thermodynamic data tables. Let's assume the following values (note: these are illustrative and might not be exactly precise):
- ΔHf°(CH₄(g)) = -74.8 kJ/mol
- ΔHf°(O₂(g)) = 0 kJ/mol (element in its standard state)
- ΔHf°(CO₂(g)) = -393.5 kJ/mol
- ΔHf°(H₂O(l)) = -285.8 kJ/mol
Using the formula:
ΔH°<sub>rxn</sub> = [(-393.5 kJ/mol) + 2(-285.8 kJ/mol)] - [(-74.8 kJ/mol) + 2(0 kJ/mol)] ΔH°<sub>rxn</sub> = -891.1 kJ/mol
This indicates that the combustion of one mole of methane releases 891.1 kJ of heat.
2. Calorimetry
Calorimetry is an experimental method used to measure the heat absorbed or released during a reaction. A calorimeter is a device that measures the temperature change resulting from a chemical or physical process. By knowing the heat capacity of the calorimeter and the temperature change, we can calculate the heat exchanged, which is equal to ΔH at constant pressure.
The basic formula used in calorimetry is:
q = mcΔT
Where:
- q is the heat transferred (in Joules)
- m is the mass of the substance (in grams)
- c is the specific heat capacity of the substance (in J/g°C)
- ΔT is the change in temperature (in °C)
At constant pressure, q = ΔH. For more sophisticated calorimeters, corrections for heat loss to the surroundings might be needed.
3. Hess's Law
Hess's Law states that the enthalpy change for a reaction is independent of the pathway taken. This means that if a reaction can be expressed as a sum of several steps, the overall enthalpy change is the sum of the enthalpy changes for each step. This is particularly useful when the direct measurement of ΔH is difficult or impossible.
Example:
Consider a reaction A → C, where the direct enthalpy change is unknown. If we can find two reactions:
A → B ΔH₁ B → C ΔH₂
Then, the enthalpy change for A → C is ΔH₁ + ΔH₂.
4. Bond Energies
Bond energy is the enthalpy change required to break one mole of a specific type of bond in the gaseous state. By knowing the bond energies of the bonds broken and formed in a reaction, we can estimate the enthalpy change.
The formula is:
ΔH°<sub>rxn</sub> = Σ [Bond energies of bonds broken] - Σ [Bond energies of bonds formed]
5. Using Standard Enthalpies of Combustion (ΔHc°)
Similar to standard enthalpies of formation, standard enthalpies of combustion are tabulated values representing the enthalpy change when one mole of a substance undergoes complete combustion in oxygen. This method is particularly useful for organic compounds. The calculation involves a similar approach to using standard enthalpies of formation, but with combustion reactions as the basis.
Practical Considerations and Advanced Topics
While the methods described above provide a solid foundation for determining ΔH, several practical considerations and advanced topics warrant attention:
- Standard conditions: ΔH values are often reported under standard conditions (298 K and 1 atm). Deviations from these conditions require adjustments using thermodynamic principles.
- Temperature dependence: ΔH can vary with temperature. Kirchhoff's Law provides a way to estimate the change in ΔH with temperature, using the heat capacities of the reactants and products.
- Phase transitions: Enthalpy changes associated with phase transitions (e.g., melting, boiling) must be considered when reactants or products undergo these changes.
- Accuracy and uncertainties: Experimental measurements, such as those from calorimetry, always involve uncertainties. Proper error analysis is crucial for reliable results.
- Complex reactions: For complex reactions with multiple steps, more sophisticated thermodynamic models might be necessary.
Conclusion
Determining the change in enthalpy is a cornerstone of thermochemistry, providing valuable insights into the energy changes accompanying chemical and physical processes. This guide covered several methods for calculating ΔH, from using standard enthalpy data to employing experimental techniques like calorimetry. Understanding these methods and their limitations is crucial for anyone working in fields involving thermodynamics and chemical reactions. Remember to always consider the context of the problem and choose the most appropriate method for accurately determining the change in enthalpy. The careful consideration of practical aspects, such as standard conditions and potential sources of error, ensures the reliability and meaningfulness of the results obtained.
Latest Posts
Latest Posts
-
Does Reduction Happen At The Cathode
Mar 25, 2025
-
What Are The Units For Wavelength
Mar 25, 2025
-
What Instrument Is Used For Measuring Mass
Mar 25, 2025
-
Electric Field Between Two Opposite Charges
Mar 25, 2025
-
In A Molecule With Covalent Bonding
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Change In Enthalpy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
