In A Molecule With Covalent Bonding
Muz Play
Mar 25, 2025 · 7 min read
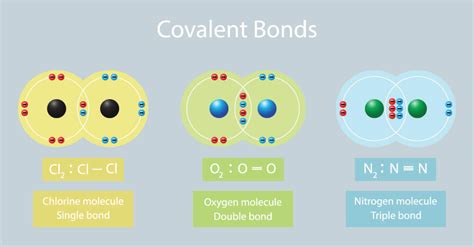
Table of Contents
In a Molecule with Covalent Bonding: A Deep Dive into Shared Electrons
Covalent bonding, a fundamental concept in chemistry, forms the bedrock of countless molecules, shaping the properties and interactions of matter as we know it. Understanding covalent bonding is crucial for comprehending the structure and behavior of everything from simple water molecules to complex biological macromolecules like DNA. This article delves into the intricacies of covalent bonding, exploring its nature, types, properties, and implications in various chemical contexts.
The Essence of Covalent Bonding: Sharing is Caring
Unlike ionic bonding, where electrons are transferred from one atom to another, covalent bonding involves the sharing of electrons between atoms. This sharing occurs to achieve a stable electron configuration, typically resembling that of a noble gas (a full outer electron shell). Atoms with similar electronegativities, meaning they have a comparable pull on electrons, are most likely to engage in covalent bonding. This often occurs between nonmetal atoms.
The Formation of a Covalent Bond
The process begins when two or more atoms approach each other. Their valence electrons, the outermost electrons involved in chemical bonding, interact. If the atoms have unpaired electrons in their valence shells, these electrons can pair up, forming a shared electron pair, or a covalent bond. This shared pair of electrons is attracted to the nuclei of both atoms, holding them together.
Representing Covalent Bonds: Lewis Structures and Molecular Formulas
Several methods represent covalent bonds:
-
Lewis Structures (Lewis Dot Diagrams): These diagrams depict the valence electrons as dots around the atom's symbol. Shared electron pairs are shown as lines connecting the atoms. Lewis structures provide a visual representation of the bonding and lone pairs (unshared electrons) within a molecule.
-
Molecular Formulas: These formulas show the types and numbers of atoms in a molecule. For example, H₂O represents a water molecule with two hydrogen atoms and one oxygen atom. While providing the elemental composition, molecular formulas don't reveal the bonding arrangement.
-
Structural Formulas: These formulas depict the arrangement of atoms and bonds within a molecule. They offer a more detailed picture than molecular formulas, showing single, double, or triple bonds.
Types of Covalent Bonds: Single, Double, and Triple Bonds
The number of shared electron pairs determines the bond order and influences the bond strength and length:
-
Single Covalent Bond: Involves one shared electron pair (one line in Lewis structures). These bonds are relatively weak and long. Example: The C-H bond in methane (CH₄).
-
Double Covalent Bond: Involves two shared electron pairs (two lines in Lewis structures). These bonds are stronger and shorter than single bonds. Example: The C=O bond in carbon dioxide (CO₂).
-
Triple Covalent Bond: Involves three shared electron pairs (three lines in Lewis structures). These bonds are the strongest and shortest. Example: The C≡N bond in hydrogen cyanide (HCN).
Polarity in Covalent Bonds: Electronegativity Differences
While covalent bonds involve sharing, the sharing isn't always equal. Electronegativity, the ability of an atom to attract electrons in a bond, plays a crucial role. If atoms have different electronegativities, the shared electrons are pulled more strongly towards the more electronegative atom, creating a polar covalent bond.
Nonpolar Covalent Bonds
When atoms have similar or identical electronegativities (e.g., in a diatomic molecule like O₂ or Cl₂), the electron sharing is relatively even, resulting in a nonpolar covalent bond. The electron density is equally distributed.
Polar Covalent Bonds
When atoms have significantly different electronegativities (e.g., in a molecule like HCl), the electrons are pulled more towards the more electronegative atom (in this case, Cl). This creates a polar covalent bond, with a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom. This uneven distribution of charge results in a dipole moment.
The Impact of Covalent Bonding on Molecular Properties
The type of covalent bond (single, double, triple) and its polarity significantly influence a molecule's properties:
-
Bond Strength and Length: Triple bonds are stronger and shorter than double bonds, which are stronger and shorter than single bonds. This influences the molecule's reactivity and stability.
-
Melting and Boiling Points: Molecules with stronger covalent bonds typically have higher melting and boiling points. Polar molecules also tend to have higher melting and boiling points due to stronger intermolecular forces (dipole-dipole interactions, hydrogen bonding).
-
Solubility: Polar molecules tend to dissolve in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents (like oil). This is due to the principle of "like dissolves like."
-
Reactivity: The presence of double or triple bonds, or polar bonds, can make a molecule more reactive. These bonds often act as sites for chemical reactions.
Covalent Bonding in Organic Chemistry
Covalent bonding is the foundation of organic chemistry, the study of carbon-containing compounds. Carbon's ability to form four covalent bonds allows for the creation of diverse and complex organic molecules, including:
-
Hydrocarbons: These molecules contain only carbon and hydrogen atoms, forming the basis of many fuels and organic materials. Examples include methane (CH₄), ethane (C₂H₆), and benzene (C₆H₆).
-
Functional Groups: These are specific groups of atoms within organic molecules that impart characteristic properties. Examples include hydroxyl groups (-OH), carboxyl groups (-COOH), and amino groups (-NH₂). These groups influence the molecule's reactivity and behavior.
-
Macromolecules: These are large, complex molecules essential for life. Examples include proteins, carbohydrates, lipids, and nucleic acids (DNA and RNA), all built upon frameworks of covalent bonds. The specific sequence of covalent bonds determines the macromolecule's structure and function.
Covalent Bonding and Intermolecular Forces
While covalent bonds hold atoms together within a molecule, intermolecular forces attract molecules to each other. These forces are weaker than covalent bonds but play a crucial role in determining the physical properties of substances:
-
London Dispersion Forces: These weak forces exist between all molecules, resulting from temporary fluctuations in electron distribution.
-
Dipole-Dipole Interactions: These forces occur between polar molecules, due to the attraction between the partially positive and partially negative ends of the molecules.
-
Hydrogen Bonding: A special type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. Hydrogen bonding is particularly strong and influences the properties of water and many biological molecules.
Beyond the Basics: Advanced Concepts in Covalent Bonding
This section touches on more advanced concepts related to covalent bonding:
-
Resonance Structures: Some molecules cannot be accurately represented by a single Lewis structure. Instead, they exhibit resonance, meaning the electron density is delocalized across multiple bonds. Benzene is a classic example.
-
Coordinate Covalent Bonds (Dative Bonds): In these bonds, both electrons in the shared pair originate from the same atom. This is often observed in the formation of complex ions.
-
Bond Energies and Enthalpies: The energy required to break a covalent bond is its bond energy or bond enthalpy. These values are crucial in thermodynamics and reaction kinetics.
-
Bond Length and Bond Angles: These parameters provide insights into the molecular geometry and shape, which influence reactivity and properties. VSEPR (Valence Shell Electron Pair Repulsion) theory helps predict these geometric aspects.
-
Hybridization: This concept explains how atomic orbitals combine to form hybrid orbitals that are involved in covalent bonding. For example, carbon's sp³, sp², and sp hybridized orbitals explain the geometries of different organic molecules.
Conclusion: The Ubiquity of Covalent Bonds
Covalent bonding is a fundamental force governing the structure and properties of a vast array of molecules. From simple diatomic gases to complex biological macromolecules, the sharing of electrons forms the basis of chemical interactions and dictates the behavior of matter. A thorough understanding of covalent bonding is essential for comprehending chemistry in all its diverse manifestations, from the synthesis of new materials to understanding the intricate mechanisms of life itself. Further exploration of the concepts discussed above, including the application of advanced techniques and theoretical models, provides a richer and more complete picture of this vital chemical phenomenon.
Latest Posts
Latest Posts
-
Factoring The Greatest Common Monomial Factor
Mar 26, 2025
-
Vascular Anatomy Of The Lower Extremity
Mar 26, 2025
-
Bohr Diagrams Of The First 20 Elements
Mar 26, 2025
-
Comparison Test For Convergence And Divergence
Mar 26, 2025
-
What Is Pathophysiology Of A Disease
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about In A Molecule With Covalent Bonding . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
