Does Reduction Happen At The Cathode
Muz Play
Mar 25, 2025 · 5 min read
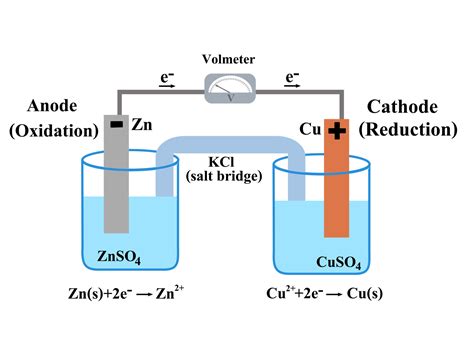
Table of Contents
Does Reduction Happen at the Cathode? Understanding Redox Reactions in Electrochemistry
Electrochemistry, the study of the relationship between chemical reactions and electrical energy, hinges on the concepts of oxidation and reduction. These processes, collectively known as redox reactions, are fundamental to numerous applications, from batteries and fuel cells to corrosion and electroplating. A common question that arises, particularly for beginners, is: does reduction happen at the cathode? The short answer is a resounding yes. However, understanding why this is the case requires delving into the intricacies of electrochemical cells and the definitions of oxidation and reduction.
Understanding Oxidation and Reduction
Before we pinpoint the location of reduction in an electrochemical cell, let's solidify our understanding of the core concepts:
Oxidation: Loss of Electrons
Oxidation is defined as the loss of electrons by an atom, ion, or molecule. This process results in an increase in the oxidation state of the species involved. Consider the simple example of the oxidation of iron:
Fe → Fe²⁺ + 2e⁻
In this reaction, a neutral iron atom loses two electrons to become a positively charged iron(II) ion. The iron atom is said to be oxidized.
Reduction: Gain of Electrons
Reduction, conversely, is the gain of electrons. This process results in a decrease in the oxidation state of the species. Returning to the iron example, if we were to reverse the process, we would have reduction:
Fe²⁺ + 2e⁻ → Fe
Here, the iron(II) ion gains two electrons to become a neutral iron atom. The iron ion is said to be reduced.
The Redox Couple
It's crucial to recognize that oxidation and reduction always occur simultaneously. You cannot have one without the other. The species undergoing oxidation and reduction together form a redox couple. In our iron example, the redox couple is Fe²⁺/Fe.
Electrochemical Cells: A Closer Look
Electrochemical cells are devices that harness the energy released from spontaneous redox reactions or use electrical energy to drive non-spontaneous redox reactions. They consist of two electrodes, an anode and a cathode, immersed in an electrolyte solution.
The Anode: Site of Oxidation
The anode is the electrode where oxidation occurs. Electrons are released from the species undergoing oxidation at the anode. These electrons then flow through an external circuit to the cathode. Because the anode is the source of electrons, it is considered the negative electrode in a galvanic cell (a cell that produces electrical energy).
The Cathode: Site of Reduction
The cathode is the electrode where reduction occurs. Electrons flow from the external circuit to the cathode, where they are accepted by the species undergoing reduction. Since the cathode is where electrons are consumed, it is considered the positive electrode in a galvanic cell.
Electrolytic Cells vs. Galvanic Cells
The designation of the anode and cathode as positive or negative depends on the type of electrochemical cell:
-
Galvanic Cells (Voltaic Cells): These cells generate electrical energy from a spontaneous redox reaction. The anode is negative, and the cathode is positive. Electrons flow spontaneously from the anode to the cathode.
-
Electrolytic Cells: These cells use electrical energy to drive a non-spontaneous redox reaction. The anode is positive, and the cathode is negative. An external power source forces electrons to flow from the cathode to the anode, driving the otherwise unfavorable reaction.
Regardless of the cell type, reduction always occurs at the cathode. The flow of electrons is what determines the polarity in galvanic cells, but the fundamental redox process remains consistent.
Examples Illustrating Reduction at the Cathode
Let's examine some practical examples to further solidify the understanding of reduction at the cathode:
1. The Zinc-Copper Cell (Daniell Cell)
This classic galvanic cell comprises a zinc anode (Zn → Zn²⁺ + 2e⁻) and a copper cathode (Cu²⁺ + 2e⁻ → Cu). The zinc electrode is oxidized, releasing electrons that flow through the external circuit to the copper electrode. At the copper cathode, copper(II) ions from the solution accept these electrons and are reduced to metallic copper.
2. Electroplating
Electroplating is a process used to coat a metal object with a thin layer of another metal. This is an electrolytic process. The object to be plated acts as the cathode. Metal ions from a solution containing the desired plating metal are reduced at the cathode, depositing the metal onto the object's surface. For example, in chromium plating, chromium(III) ions (Cr³⁺) are reduced to metallic chromium (Cr) at the cathode.
3. Battery Operation
Batteries, both primary (non-rechargeable) and secondary (rechargeable), rely on redox reactions to generate electrical energy. Reduction always takes place at the cathode during discharge (in primary batteries) or when the battery is being used (in secondary batteries). For instance, in a lithium-ion battery, lithium ions (Li⁺) are reduced at the cathode during discharge, integrating into the cathode material.
Mnemonic Devices and Further Clarification
Remembering that reduction happens at the cathode can be aided by mnemonics. A popular one is "Red Cat": Reduction at the Cathode. Another is "An Ox": Anode is where Oxidation occurs.
It's also important to reiterate that the terms anode and cathode refer to the electrodes' roles in the redox reaction, not their inherent electrical charge. While in a galvanic cell the anode is negative and the cathode is positive, this is reversed in an electrolytic cell. However, the fundamental processes of oxidation at the anode and reduction at the cathode remain constant.
Conclusion: The Cathode as the Reduction Hub
In conclusion, the statement "reduction happens at the cathode" is a fundamental principle in electrochemistry. This applies regardless of whether the electrochemical cell is galvanic or electrolytic. Understanding this principle is crucial for comprehending the workings of various electrochemical devices, from batteries and fuel cells to industrial processes like electroplating and metal refining. By grasping the concepts of oxidation and reduction, and their association with the anode and cathode, one can unlock a deeper appreciation for the fascinating world of electrochemistry and its widespread applications. The consistent occurrence of reduction at the cathode underscores its critical role in the transfer of electrons and the driving force behind numerous chemical transformations.
Latest Posts
Latest Posts
-
The Diels Alder Reaction Is A Concerted Reaction Define Concerted
Mar 26, 2025
-
How Many Fatty Acids Are Needed To Form A Glycerophospholipid
Mar 26, 2025
-
What Type Of Distortion Does The Good Homolosine Preserve
Mar 26, 2025
-
Autotrophs Make Their Own Food Using Energy From
Mar 26, 2025
-
Leave As Is To A Writer
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Does Reduction Happen At The Cathode . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
