The Diels Alder Reaction Is A Concerted Reaction. Define Concerted.
Muz Play
Mar 26, 2025 · 7 min read
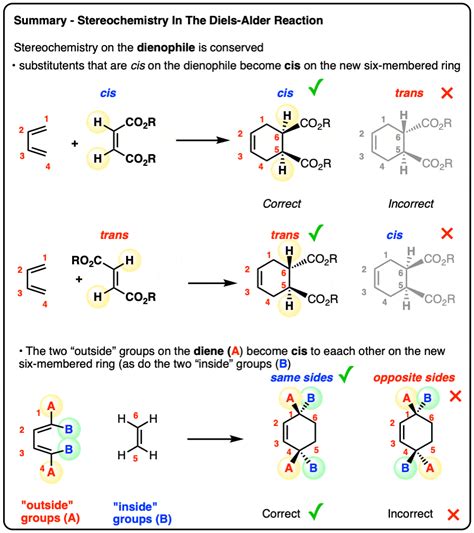
Table of Contents
The Diels-Alder Reaction: A Deep Dive into Concerted Cycloadditions
The Diels-Alder reaction, a cornerstone of organic chemistry, stands as a testament to the elegance and efficiency of concerted pericyclic reactions. Its ability to construct six-membered rings in a single step has made it invaluable in the synthesis of complex molecules, from pharmaceuticals to natural products. Understanding the reaction's mechanism, particularly its concerted nature, is crucial to harnessing its full potential. This article will delve into the intricacies of the Diels-Alder reaction, providing a comprehensive explanation of what it means for a reaction to be concerted, and exploring the implications of this characteristic.
What Does "Concerted" Mean in a Chemical Reaction?
Before diving into the specifics of the Diels-Alder reaction, let's define the key term: concerted. In the context of chemical reactions, a concerted reaction is one that proceeds in a single step, without the formation of any intermediates. This means that all bond breaking and bond forming occurs simultaneously, in a synchronized fashion. There's no build-up of charge, no discrete carbocation or carbanion intermediates, and no distinct steps separable in time. The reactants transform directly into products through a single transition state.
This contrasts sharply with stepwise reactions, which involve distinct, identifiable intermediates. For example, many SN1 reactions proceed through a carbocation intermediate, while SN2 reactions often involve a pentacoordinate transition state but still follow a stepwise progression of bond breaking and bond making. The concerted nature of a reaction dramatically influences its stereochemistry and regiochemistry.
The Diels-Alder Reaction: A Classic Concerted Cycloaddition
The Diels-Alder reaction is a [4+2] cycloaddition, meaning a four-carbon component (the diene) reacts with a two-carbon component (the dienophile) to form a six-membered ring. Crucially, this transformation occurs in a concerted manner. The diene adopts an s-cis conformation (meaning the two double bonds are on the same side), and simultaneously interacts with the dienophile. The π-electrons of both the diene and dienophile rearrange to form two new σ-bonds and one new π-bond, all in one coordinated movement.
Visualization of the Concerted Mechanism
Imagine the process like a synchronized dance: the diene and dienophile approach each other, their electron clouds interacting. As the bonds begin to form, the old π-bonds break simultaneously, creating a seamless transition from reactants to products. This single, concerted step is reflected in the single transition state, a high-energy configuration where old bonds are partially broken and new bonds are partially formed.
Evidence Supporting the Concerted Mechanism
Several lines of experimental evidence strongly support the concerted mechanism of the Diels-Alder reaction:
-
Stereospecificity: The stereochemistry of the reactants is largely preserved in the product. This observation is consistent with a concerted mechanism, where the bond formations occur simultaneously and prevent any rotation or rearrangement that would lead to loss of stereochemical information. A stepwise mechanism, on the other hand, would allow for rotation around bonds before the final product is formed, leading to a mixture of stereoisomers.
-
Kinetic Isotope Effects: Studies involving deuterium labeling have revealed minimal kinetic isotope effects, meaning the rate of the reaction is not significantly affected by replacing hydrogen atoms with deuterium. This observation is consistent with a concerted mechanism, where the C-H bonds are not significantly broken or formed in the rate-determining step. A stepwise mechanism, involving significant C-H bond breaking or formation in the rate-limiting step, would exhibit a larger kinetic isotope effect.
-
Absence of Intermediates: No intermediates have been detected during the reaction, further bolstering the argument for a concerted mechanism. If intermediates existed, they would likely be detectable through spectroscopic techniques or other experimental methods.
-
Theoretical Calculations: Advanced computational methods provide strong theoretical support for the concerted nature of the reaction, showing the existence of a single transition state connecting the reactants and products.
Factors Influencing the Diels-Alder Reaction
Several factors can influence the rate and stereoselectivity of the Diels-Alder reaction:
-
Electron-withdrawing groups on the dienophile: Electron-withdrawing groups on the dienophile enhance the reaction rate by increasing the electrophilicity of the dienophile. This makes it more susceptible to nucleophilic attack by the diene.
-
Electron-donating groups on the diene: Electron-donating groups on the diene enhance the reaction rate by increasing the nucleophilicity of the diene. This makes it more reactive towards the electrophilic dienophile.
-
Solvent effects: Polar solvents generally accelerate the Diels-Alder reaction by stabilizing the polar transition state.
-
Temperature: Higher temperatures generally favor the reaction, particularly for reactions with high activation energies.
-
Pressure: High pressure can also accelerate the reaction by favoring the formation of the more compact cyclic product.
Regioselectivity and Stereoselectivity in Diels-Alder Reactions
The concerted nature of the Diels-Alder reaction has profound consequences for its regio- and stereoselectivity. Regioselectivity refers to the preferential formation of one regioisomer over another, while stereoselectivity refers to the preferential formation of one stereoisomer over another.
Regioselectivity: The "ortho" and "para" directing effects
The regioselectivity of the Diels-Alder reaction is governed by the electronic effects of substituents on the diene and dienophile. Electron-donating groups on the diene and electron-withdrawing groups on the dienophile often lead to the formation of the para product, while the opposite electronic arrangement favors the ortho product. This is because the transition state leading to the preferred product is energetically more favorable, due to better orbital overlap and charge stabilization.
Stereoselectivity: Endo vs. Exo
In the Diels-Alder reaction between cyclic dienes and dienophiles, the stereoselectivity is often influenced by the endo rule. This empirical rule states that the endo isomer (where the substituents on the dienophile are oriented towards the newly formed bridgehead carbons) is preferentially formed over the exo isomer (where the substituents are oriented away from the bridgehead carbons). This preference is attributed to secondary orbital interactions between the π-system of the dienophile and the π-system of the diene in the transition state.
However, this rule is not absolute, and other factors, such as steric hindrance, can override the endo preference. The endo/exo selectivity often depends on the balance between electronic and steric effects in the transition state.
Applications of the Diels-Alder Reaction
The Diels-Alder reaction's versatility and efficiency have established it as a powerful tool in organic synthesis. Its applications are vast and span numerous fields:
-
Natural product synthesis: The Diels-Alder reaction is frequently employed in the synthesis of complex natural products, particularly those containing six-membered rings. Its ability to create stereochemical centers in a controlled manner makes it invaluable in the construction of chiral molecules.
-
Medicinal chemistry: This reaction plays a crucial role in the synthesis of pharmaceuticals, allowing for the efficient construction of drug scaffolds with diverse functionalities.
-
Polymer chemistry: The Diels-Alder reaction is utilized in the preparation of polymers with specific properties. The reaction's reversibility under certain conditions can even lead to the formation of self-healing polymers.
-
Material science: The reaction is used in creating new materials with tailored properties, including advanced composites and functional polymers.
Conclusion: The Significance of Concertedness
The concerted nature of the Diels-Alder reaction is a defining characteristic that dictates its remarkable efficiency, stereospecificity, and regioselectivity. This single-step transformation avoids the formation of high-energy intermediates, allowing for the rapid and controlled construction of complex molecules. Understanding the concerted mechanism and the factors influencing it is crucial for synthetic chemists to design and execute efficient Diels-Alder reactions, ultimately leading to the synthesis of valuable molecules with applications across various fields of science and technology. The reaction’s significance continues to grow, as researchers continue to explore its nuances and exploit its potential in the creation of new materials and medicines. Further research into the intricacies of this pericyclic reaction promises to unlock even greater potential for its application in organic synthesis and beyond.
Latest Posts
Latest Posts
-
Rate Of Change Positive And Decreasing
Mar 26, 2025
-
A Chemical Reaction Is At Equilibrium When
Mar 26, 2025
-
Changing The Order Of Integration Triple Integrals
Mar 26, 2025
-
What Is The Principle Of Cross Cutting Relationships
Mar 26, 2025
-
Limits At Infinity And Infinite Limits
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about The Diels Alder Reaction Is A Concerted Reaction. Define Concerted. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
