A Chemical Reaction Is At Equilibrium When
Muz Play
Mar 26, 2025 · 6 min read
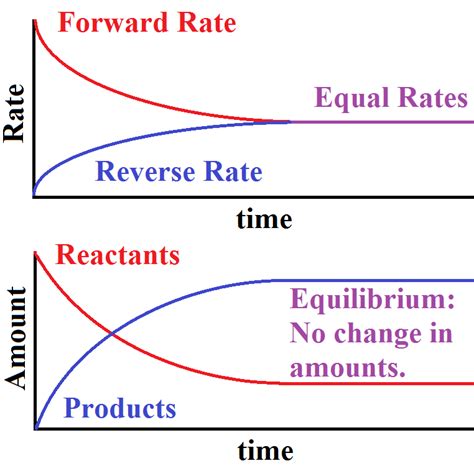
Table of Contents
A Chemical Reaction is at Equilibrium When… Understanding the Dynamic Balance
Chemical equilibrium is a fundamental concept in chemistry, crucial for understanding countless processes in nature and industry. It's not a static state, as many initially assume, but rather a dynamic balance where the rates of the forward and reverse reactions are equal. This article delves deep into the intricacies of chemical equilibrium, exploring its characteristics, influencing factors, and practical applications.
What is Chemical Equilibrium?
A chemical reaction is at equilibrium when the rates of the forward and reverse reactions are equal. This doesn't mean the concentrations of reactants and products are equal; rather, it signifies that the net change in their concentrations is zero. Imagine a crowded marketplace: people are constantly entering and leaving, but the overall number remains relatively constant. Equilibrium is similar – molecules are continuously transforming between reactants and products, but the overall amounts remain unchanged.
The Dynamic Nature of Equilibrium
It's crucial to emphasize the dynamic aspect of equilibrium. The reactions continue to occur; they haven't stopped. The forward reaction (reactants forming products) and the reverse reaction (products forming reactants) proceed at the same pace, resulting in no observable net change. This is a state of balance, not a state of inactivity.
Representing Equilibrium: The Double Arrow
In chemical equations, equilibrium is represented using a double arrow (⇌). This symbolizes the bidirectional nature of the reaction. For instance:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
This equation shows the reversible reaction between nitrogen and hydrogen to form ammonia. The double arrow indicates that the reaction proceeds in both directions simultaneously at equilibrium.
Factors Affecting Chemical Equilibrium: Le Chatelier's Principle
The position of equilibrium – the relative amounts of reactants and products – can be shifted by altering certain conditions. Le Chatelier's principle provides a framework for understanding these shifts: If a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress.
1. Concentration Changes
Changing the concentration of reactants or products will disturb the equilibrium. If we increase the concentration of a reactant, the equilibrium will shift to the right, favoring the forward reaction and producing more products. Conversely, increasing the concentration of a product will shift the equilibrium to the left, favoring the reverse reaction and producing more reactants.
Example: Consider the Haber-Bosch process for ammonia synthesis (N₂(g) + 3H₂(g) ⇌ 2NH₃(g)). Increasing the concentration of nitrogen (N₂) will drive the equilibrium towards the production of more ammonia (NH₃).
2. Temperature Changes
Temperature changes affect the equilibrium constant (K), a value that quantifies the relative amounts of reactants and products at equilibrium. The effect of temperature depends on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat).
-
Exothermic Reactions: Increasing the temperature favors the reverse reaction (absorbing heat), shifting the equilibrium to the left. Decreasing the temperature favors the forward reaction (releasing heat), shifting the equilibrium to the right.
-
Endothermic Reactions: Increasing the temperature favors the forward reaction (absorbing heat), shifting the equilibrium to the right. Decreasing the temperature favors the reverse reaction (releasing heat), shifting the equilibrium to the left.
3. Pressure Changes
Pressure changes primarily affect equilibrium reactions involving gases. Increasing the pressure favors the side of the reaction with fewer moles of gas, while decreasing the pressure favors the side with more moles of gas.
Example: In the Haber-Bosch process, the forward reaction (N₂(g) + 3H₂(g) ⇌ 2NH₃(g)) has fewer moles of gas (2 moles of NH₃) than the reactants (4 moles of N₂ and H₂). Increasing the pressure will therefore shift the equilibrium to the right, favoring ammonia production.
4. Addition of a Catalyst
A catalyst speeds up both the forward and reverse reactions equally. It does not affect the position of equilibrium; it only helps the system reach equilibrium faster. The equilibrium constant (K) remains unchanged.
The Equilibrium Constant (K)
The equilibrium constant (K) is a numerical value that describes the relative amounts of reactants and products at equilibrium. It's a ratio of the concentrations of products to the concentrations of reactants, each raised to the power of its stoichiometric coefficient in the balanced chemical equation.
For a general reaction: aA + bB ⇌ cC + dD
The equilibrium constant expression is:
K = ([C]ᶜ[D]ᵈ) / ([A]ᵃ[B]ᵇ)
where [A], [B], [C], and [D] represent the equilibrium concentrations of the respective species.
The value of K indicates the extent to which the reaction proceeds to completion at equilibrium:
- K >> 1: The equilibrium lies far to the right; the reaction strongly favors product formation.
- K ≈ 1: The equilibrium lies roughly in the middle; significant amounts of both reactants and products are present.
- K << 1: The equilibrium lies far to the left; the reaction strongly favors reactant formation.
Applications of Chemical Equilibrium
Chemical equilibrium principles are essential across various fields:
-
Industrial Processes: Optimizing industrial processes, such as the Haber-Bosch process for ammonia synthesis, requires a deep understanding of equilibrium to maximize product yield. Adjusting temperature, pressure, and reactant concentrations can significantly influence the efficiency of these processes.
-
Environmental Chemistry: Equilibrium concepts are crucial in understanding environmental processes, like the distribution of pollutants in water and air, acid-base equilibria in natural waters, and the solubility of minerals.
-
Biochemistry: Biochemical reactions within living organisms are governed by equilibrium principles. Understanding enzyme kinetics and the equilibrium constants of biochemical reactions is fundamental to comprehending metabolic pathways and cellular processes.
-
Analytical Chemistry: Equilibrium constants are used extensively in analytical chemistry for developing quantitative methods for determining the concentrations of various substances. Examples include acid-base titrations, complexometric titrations, and solubility product calculations.
-
Medicine: Many pharmaceutical drugs function by interacting with biological molecules, often through reversible binding. Understanding the equilibrium constants of these interactions is crucial for drug development and design.
Beyond Simple Equilibria: Complex Systems
While this article focuses on simple equilibrium systems, the principles extend to more complex scenarios, including:
-
Simultaneous Equilibria: Systems with multiple simultaneous equilibria require considering the interactions between different reactions.
-
Heterogeneous Equilibria: These involve reactants and products in different phases (e.g., solid, liquid, gas), requiring modification of the equilibrium constant expression to include activities or partial pressures instead of concentrations.
-
Ionic Equilibria: These focus on the equilibrium of ions in solution, including acid-base equilibria, solubility equilibria, and complex ion formation. Understanding these is critical for many chemical and biological processes.
Conclusion: A Dynamic Balance Shaping Our World
Chemical equilibrium is not a static endpoint but a dynamic balance reflecting the continuous interplay between forward and reverse reactions. Le Chatelier's principle helps predict how changes in conditions will affect this balance. The equilibrium constant (K) quantifies the extent of the reaction at equilibrium, offering valuable insight into reaction progress and product yields. Mastering chemical equilibrium is essential across numerous scientific and industrial domains, shaping our understanding of natural processes and guiding the design of efficient technological systems. From industrial ammonia production to the delicate balance of biochemical reactions within our bodies, the principle of equilibrium remains a cornerstone of modern chemistry.
Latest Posts
Latest Posts
-
Calculating An Equilibrium Constant From A Heterogeneous Equilibrium Composition
Mar 29, 2025
-
Atomic Structure Of Elements In Periodic Table
Mar 29, 2025
-
Give The Iupac Name For This Alkane
Mar 29, 2025
-
Difference Between Alcoholic And Lactic Acid Fermentation
Mar 29, 2025
-
2 Letter Symbols From The Periodic Table
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about A Chemical Reaction Is At Equilibrium When . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
