Give The Iupac Name For This Alkane.
Muz Play
Mar 29, 2025 · 6 min read
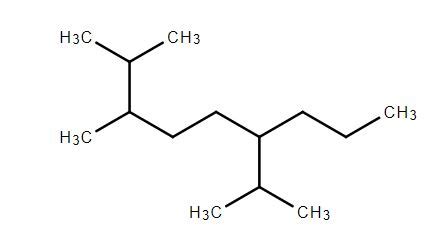
Table of Contents
Give the IUPAC Name for This Alkane: A Comprehensive Guide to Alkane Nomenclature
Alkanes, the simplest of hydrocarbons, form the backbone of organic chemistry. Understanding their nomenclature, governed by the International Union of Pure and Applied Chemistry (IUPAC), is crucial for any aspiring chemist. This comprehensive guide will delve into the intricacies of naming alkanes, equipping you with the skills to confidently assign IUPAC names to even the most complex structures. We'll explore the fundamental rules, tackle common challenges, and provide numerous examples to solidify your understanding.
Understanding the Fundamentals of Alkane Nomenclature
Before diving into complex structures, let's lay the groundwork. Alkanes are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms and are bonded to hydrogen atoms to satisfy carbon's valency. The general formula for an alkane is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms.
The simplest alkane is methane (CH<sub>4</sub>), followed by ethane (C<sub>2</sub>H<sub>6</sub>), propane (C<sub>3</sub>H<sub>8</sub>), and butane (C<sub>4</sub>H<sub>10</sub>). These are the foundational names you'll build upon when naming more complex alkanes.
Key Principles of IUPAC Nomenclature:
-
Finding the Parent Chain: Identify the longest continuous chain of carbon atoms in the molecule. This chain forms the basis of the alkane's name. This is crucial, even if it means ignoring seemingly shorter branches that might be more visually prominent.
-
Numbering the Carbon Atoms: Number the carbon atoms in the parent chain. Start numbering from the end that gives the substituents (alkyl groups) the lowest possible numbers. The goal is to achieve the lowest set of locants (numbers).
-
Identifying Substituents: Any carbon atoms branching off from the parent chain are considered substituents or alkyl groups. These are named by replacing the '-ane' ending of the corresponding alkane with '-yl'. For example, a methyl group (-CH₃) comes from methane, an ethyl group (-CH₂CH₃) from ethane, and so on.
-
Naming the Substituents: List the substituents alphabetically, ignoring any prefixes like 'di-', 'tri-', or 'tetra-' (except for iso-, sec-, and tert- which are considered part of the alkyl group name itself).
-
Indicating the Position of Substituents: Use the numbers assigned to the parent chain to indicate the position of each substituent. These numbers are called locants. Place these numbers immediately before the name of the substituent.
-
Multiple Substituents of the Same Type: If multiple substituents of the same type are present, use prefixes like 'di-', 'tri-', 'tetra-', etc., to indicate their number. Separate these numbers with commas and use hyphens to connect numbers to words.
-
Combining the Information: Combine the information to create the complete IUPAC name. The name follows the order: (locant)-(substituent)-(locant)-(substituent)-...-(parent alkane).
Working Through Examples: A Step-by-Step Approach
Let's apply these principles to several examples, starting with simpler structures and progressing to more complex ones.
Example 1:
! (Imagine a simple branched alkane with a methyl group on the second carbon of a propane chain)
-
Parent Chain: The longest continuous chain contains three carbon atoms, making the parent alkane propane.
-
Numbering: Number the carbon atoms from the end closest to the methyl group (giving the methyl group the lowest locant).
-
Substituent: The substituent is a methyl group (-CH₃).
-
Combining: The IUPAC name is 2-methylpropane.
Example 2:
! (Imagine an alkane with two methyl groups, one on carbon 2 and one on carbon 3 of a butane chain)
-
Parent Chain: The longest continuous chain has four carbons, making it butane.
-
Numbering: Numbering from either end gives the same locants for the substituents.
-
Substituents: Two methyl groups are present.
-
Combining: The IUPAC name is 2,3-dimethylbutane.
Example 3: A more challenging example:
! (Imagine a more complex alkane with various methyl and ethyl branches)
Let's assume this structure has a longest chain of 7 carbons with various ethyl and methyl branches at different positions. The process would be as follows:
-
Parent Chain: Identify the longest continuous chain of 7 carbons (heptane).
-
Numbering: Carefully number the chain to assign the lowest possible numbers to the substituents.
-
Substituents: Identify all substituents: methyl groups and ethyl groups.
-
Alphabetical Order: List the substituents alphabetically (ethyl before methyl).
-
Combining: Write the complete name, including locants, using hyphens and commas as needed. For example, a possible name, depending on the positions of the substituents could be: 2-ethyl-3,5-dimethylheptane, or 2,4-dimethyl-3-ethylheptane, etc. The specific name would depend on the exact positions of the substituents in the image.
Handling More Complex Scenarios
The complexity increases when dealing with more extensive branching, cyclic structures (cycloalkanes), or the presence of multiple different substituents. Let’s address some of these advanced scenarios:
Dealing with Multiple Different Substituents:
When multiple different alkyl substituents are present, list them alphabetically, irrespective of their positions. For example, an alkane with both ethyl and methyl groups would have "ethyl" listed before "methyl" in the IUPAC name, regardless of their locants.
Cycloalkanes:
Cycloalkanes are alkanes in which the carbon atoms form a closed ring. The naming convention is similar, but the prefix "cyclo-" is added before the name of the parent alkane. Substituents are numbered to give the lowest possible numbers, and they are listed alphabetically.
Isomers and Stereoisomers:
Isomers are molecules with the same molecular formula but different structural arrangements. IUPAC nomenclature distinguishes between isomers by accurately reflecting their structural differences. Stereoisomers are a special type of isomer involving spatial arrangement of atoms. While this guide focuses primarily on constitutional isomers (different bonding), understanding the role of stereochemistry is essential for naming more complex molecules.
Tips and Tricks for Mastering Alkane Nomenclature
-
Practice: The key to mastering alkane nomenclature is consistent practice. Work through numerous examples, starting with simple structures and gradually increasing the complexity.
-
Draw the Structure: If you're given a name, draw the corresponding structure. This helps solidify your understanding of how the name translates to the molecule's structure.
-
Use a Systematic Approach: Always follow the steps outlined above in a systematic manner. This minimizes errors and ensures consistency.
-
Check Your Work: Always double-check your work to ensure you've correctly identified the parent chain, numbered the carbons, and listed the substituents alphabetically.
-
Refer to IUPAC Guidelines: The official IUPAC nomenclature rules are quite extensive. Referring to the official guidelines can clarify any ambiguities or uncertainties.
Conclusion
Mastering IUPAC nomenclature for alkanes is fundamental to success in organic chemistry. By understanding and applying the principles outlined in this guide, along with consistent practice, you'll confidently assign IUPAC names to even the most complex alkane structures. Remember to always prioritize accuracy and follow the systematic approach to avoid errors. The more you practice, the more intuitive this process will become. This foundation will serve you well as you explore more complex organic molecules.
Latest Posts
Latest Posts
-
Motion Of Molecules Compared To Direction Of Motion Electromagnetic Waves
Apr 01, 2025
-
The Variance Is The Square Root Of The Standard Deviation
Apr 01, 2025
-
Easy Way To Find Common Multiples
Apr 01, 2025
-
What Is The Structural And Functional Unit Of The Kidney
Apr 01, 2025
-
Evolutionary Relationships Between Organisms Are Determined By
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Give The Iupac Name For This Alkane. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
