Difference Between Alcoholic And Lactic Acid Fermentation
Muz Play
Mar 29, 2025 · 5 min read
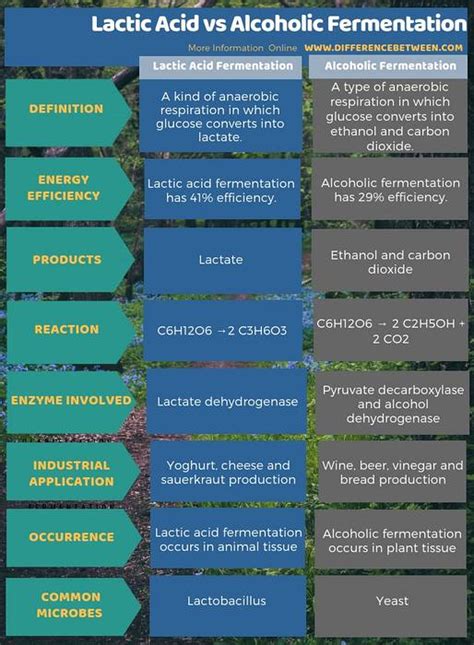
Table of Contents
- Difference Between Alcoholic And Lactic Acid Fermentation
- Table of Contents
- Delving Deep into the Differences: Alcoholic vs. Lactic Acid Fermentation
- Defining the Processes: A Foundation for Understanding
- Alcoholic Fermentation: The Essence of Brewing and Baking
- Lactic Acid Fermentation: A Muscle-Powered Process & More
- Comparing and Contrasting: Key Differences Unveiled
- Beyond the Basics: Exploring Nuances and Variations
- Variations in Alcoholic Fermentation
- Variations in Lactic Acid Fermentation
- Applications and Significance: A Broad Perspective
- Conclusion: A Continuing Story of Fermentation
- Latest Posts
- Latest Posts
- Related Post
Delving Deep into the Differences: Alcoholic vs. Lactic Acid Fermentation
Fermentation, a cornerstone of various biological processes and industrial applications, is an anaerobic process—meaning it occurs without oxygen—where microorganisms break down organic substances. While sharing the common thread of anaerobic energy production, different types of fermentation exist, each with unique characteristics and end products. This article will thoroughly explore the key differences between two prominent types: alcoholic fermentation and lactic acid fermentation. Understanding these distinctions is crucial for comprehending various biological systems and appreciating the diverse applications of fermentation in food production, biotechnology, and beyond.
Defining the Processes: A Foundation for Understanding
Both alcoholic and lactic acid fermentations are anaerobic metabolic pathways that generate energy from glucose (or other sugars) in the absence of oxygen. However, they diverge significantly in the metabolic pathways employed and the resulting byproducts.
Alcoholic Fermentation: The Essence of Brewing and Baking
Alcoholic fermentation is primarily undertaken by yeasts, single-celled fungi belonging to the genus Saccharomyces. This process begins with glycolysis, the common initial step in both types of fermentation, where glucose is broken down into pyruvate. Crucially, under anaerobic conditions, the pyruvate is subsequently converted into ethanol and carbon dioxide. This conversion involves two key steps:
- Decarboxylation: Pyruvate loses a molecule of carbon dioxide (CO2), forming acetaldehyde.
- Reduction: Acetaldehyde is reduced by NADH (nicotinamide adenine dinucleotide), a crucial electron carrier, to form ethanol. This step regenerates NAD+, which is essential for glycolysis to continue.
The net result is the production of ethanol, carbon dioxide, and a relatively small amount of ATP (adenosine triphosphate), the cell's energy currency. This process is responsible for the intoxicating effects of alcoholic beverages and the leavening action in bread making.
Lactic Acid Fermentation: A Muscle-Powered Process & More
Lactic acid fermentation is carried out by various microorganisms, including certain bacteria (like Lactobacillus and Streptococcus) and some fungi. Similar to alcoholic fermentation, it starts with glycolysis, breaking down glucose into pyruvate. However, the fate of pyruvate differs significantly. In lactic acid fermentation, pyruvate is directly reduced by NADH to form lactate (lactic acid). This single-step conversion efficiently regenerates NAD+, allowing glycolysis to proceed.
This process is crucial in various contexts:
- Muscle Metabolism: During intense exercise when oxygen supply is limited, our muscles switch to lactic acid fermentation to produce ATP. The resulting lactate buildup contributes to muscle fatigue.
- Food Production: Lactic acid fermentation is pivotal in the production of numerous fermented foods, including yogurt, sauerkraut, kimchi, and pickles. The lactic acid produced contributes to the characteristic sour taste and acts as a preservative by inhibiting the growth of spoilage microorganisms.
- Industrial Applications: Lactic acid, a valuable chemical, is also produced industrially through lactic acid fermentation for applications in food additives, biodegradable plastics, and pharmaceuticals.
Comparing and Contrasting: Key Differences Unveiled
The following table highlights the principal differences between alcoholic and lactic acid fermentation:
| Feature | Alcoholic Fermentation | Lactic Acid Fermentation |
|---|---|---|
| Organisms | Primarily yeasts (Saccharomyces) | Bacteria (Lactobacillus, Streptococcus), some fungi |
| End Products | Ethanol, Carbon Dioxide | Lactic Acid |
| Pyruvate Fate | Decarboxylation to acetaldehyde, then reduction to ethanol | Direct reduction to lactic acid |
| NADH Role | Reduces acetaldehyde to ethanol, regenerating NAD+ | Reduces pyruvate to lactic acid, regenerating NAD+ |
| ATP Yield | Relatively low | Relatively low |
| Applications | Brewing, baking, biofuel production | Food preservation (yogurt, sauerkraut, etc.), industrial lactic acid production |
| pH Change | Relatively neutral pH change | Significant decrease in pH (acidification) |
Beyond the Basics: Exploring Nuances and Variations
While the core processes described above represent the fundamental differences, nuances and variations exist within each type of fermentation:
Variations in Alcoholic Fermentation
- Different Yeast Strains: Various yeast strains exhibit different fermentation characteristics, influencing the flavor profile, aroma, and alcohol content of the final product. Some yeasts produce higher ethanol yields than others.
- Substrate Variation: Alcoholic fermentation isn't limited to glucose. Yeasts can ferment other sugars like fructose, sucrose, and maltose, adapting their metabolic pathways accordingly.
- Temperature and pH: Optimal conditions for yeast activity significantly influence fermentation efficiency and product quality.
Variations in Lactic Acid Fermentation
- Homolactic vs. Heterolactic Fermentation: Some bacteria perform homolactic fermentation, producing solely lactic acid. Others engage in heterolactic fermentation, producing lactic acid along with other byproducts like ethanol, acetic acid, and carbon dioxide. This diversity contributes to the complexity of flavors in fermented foods.
- Bacterial Species Diversity: Different lactic acid bacteria contribute distinct metabolic profiles and flavor compounds to fermented products. The selection of bacteria influences the final product's taste and texture.
- Substrate Preferences: Lactic acid bacteria can utilize a wide range of substrates beyond glucose, including other sugars and even some organic acids.
Applications and Significance: A Broad Perspective
The applications of alcoholic and lactic acid fermentation extend far beyond their roles in food and beverage production. Both processes have significant implications for:
- Biofuel Production: Alcoholic fermentation is a key technology for producing bioethanol, a renewable fuel source.
- Biotechnology: Both types of fermentation are instrumental in producing various commercially valuable chemicals and pharmaceuticals. Lactic acid, for example, serves as a precursor for biodegradable plastics.
- Waste Management: Fermentation can be used to treat organic waste, generating biogas (primarily methane) and reducing environmental impact.
- Gut Microbiome: Lactic acid bacteria play crucial roles in maintaining the health and balance of the gut microbiome, contributing to overall well-being.
Conclusion: A Continuing Story of Fermentation
Alcoholic and lactic acid fermentations, though both anaerobic metabolic pathways, present significant differences in their underlying mechanisms and final products. These differences stem from the distinct metabolic pathways employed by the microorganisms involved. While both contribute to various applications, from brewing beer and baking bread to producing yogurt and pharmaceuticals, understanding their unique characteristics is key to leveraging their full potential in diverse industrial and biological contexts. The exploration of fermentation continues to unveil new possibilities, highlighting the dynamism and importance of these fundamental biological processes in shaping our world.
Latest Posts
Latest Posts
-
Location Of Metals On The Periodic Table
Apr 02, 2025
-
What Do All Plants Have In Common
Apr 02, 2025
-
What Is The Order Of Reaction With Respect To A
Apr 02, 2025
-
Ice Melting Is A Physical Change
Apr 02, 2025
-
Elements That Have Characteristics Of Both Metals And Nonmetals
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Alcoholic And Lactic Acid Fermentation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
