Most Reactive Elements On The Periodic Table
Muz Play
Mar 23, 2025 · 6 min read
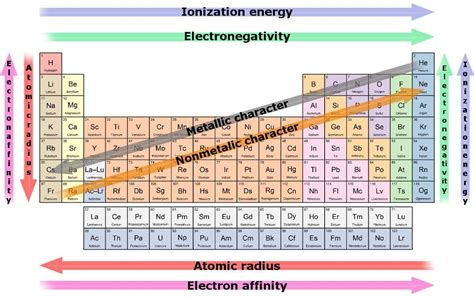
Table of Contents
The Most Reactive Elements on the Periodic Table: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Among these properties, reactivity stands out, reflecting an element's tendency to undergo chemical changes. Some elements are notoriously reactive, readily forming compounds with other elements. Understanding their reactivity is crucial in various fields, from industrial processes to biological functions. This article delves into the most reactive elements, exploring their electronic configurations, chemical behaviors, and real-world applications, while also considering safety precautions.
The Alkali Metals (Group 1): Masters of Reactivity
The alkali metals, located in Group 1 of the periodic table (lithium, sodium, potassium, rubidium, cesium, and francium), are renowned for their exceptional reactivity. This stems from their electronic configuration: they possess only one electron in their outermost shell (valence shell). This single valence electron is easily lost, resulting in the formation of a +1 cation. The ease with which they lose this electron is directly proportional to their reactivity. As you move down the group, the reactivity increases due to the increasing atomic size and decreasing ionization energy.
Lithium (Li): The Mildest Alkali Metal
While highly reactive compared to most elements, lithium is the least reactive alkali metal. Its relatively small atomic size and higher ionization energy compared to its heavier counterparts make it slightly less prone to losing its valence electron. However, it still reacts vigorously with water, producing hydrogen gas and lithium hydroxide.
Sodium (Na): Everyday Reactivity
Sodium, a common element, exemplifies the alkali metal's reactivity. Its reaction with water is quite dramatic, producing a significant amount of heat and hydrogen gas, which often ignites. Sodium is also highly reactive with halogens (Group 17), readily forming sodium halides like sodium chloride (common table salt). Its applications are vast, ranging from streetlights (sodium-vapor lamps) to the production of various chemicals.
Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr): Increasingly Fierce Reactions
Potassium, rubidium, and cesium exhibit even greater reactivity than sodium. Their reactions with water are even more vigorous, often resulting in explosions. Cesium, in particular, is extremely reactive, igniting spontaneously in air. Francium, the rarest and most unstable alkali metal, is theoretically the most reactive, but its scarcity and radioactivity limit its study. These elements are typically handled under inert atmospheres to prevent unwanted reactions.
The Alkaline Earth Metals (Group 2): A Step Down in Reactivity, But Still Significant
The alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium) in Group 2 possess two valence electrons. While less reactive than the alkali metals, they still readily lose their valence electrons to form +2 cations. Their reactivity also increases as you move down the group due to increasing atomic size and decreasing ionization energy.
Magnesium (Mg) and Calcium (Ca): Essential for Life, Reactive in Chemistry
Magnesium and calcium are crucial for biological processes. Magnesium plays a vital role in enzymatic reactions, while calcium is essential for bone structure and muscle function. Chemically, they react with water, though not as violently as alkali metals. Magnesium burns brightly in air, producing a dazzling white light – a property used in flares and fireworks. Calcium reacts more slowly with water, forming calcium hydroxide.
Strontium (Sr), Barium (Ba), and Radium (Ra): Increasing Reactivity and Radioactivity
Strontium, barium, and radium show increasingly vigorous reactions with water. Radium, like francium, is radioactive and extremely rare, limiting its practical applications. Its high radioactivity makes it extremely hazardous to handle.
The Halogens (Group 17): Electron Thieves
The halogens (fluorine, chlorine, bromine, iodine, and astatine) in Group 17 are highly reactive non-metals. They have seven valence electrons, readily gaining one electron to achieve a stable octet configuration, forming -1 anions. Their reactivity decreases down the group due to the increasing atomic size and decreasing electronegativity.
Fluorine (F): The Most Reactive Non-Metal
Fluorine is the most reactive non-metal and one of the most reactive elements overall. Its small atomic size and incredibly high electronegativity allow it to readily attract electrons from other atoms. Fluorine reacts violently with most elements, including noble gases (with the exception of helium and neon under normal conditions). It is highly corrosive and extremely dangerous to handle.
Chlorine (Cl), Bromine (Br), and Iodine (I): Reactivity Decreases Down the Group
Chlorine, bromine, and iodine exhibit decreasing reactivity compared to fluorine. Chlorine is a strong oxidizing agent and is widely used in water purification and as a disinfectant. Bromine is a liquid at room temperature, and iodine is a solid with a characteristic purple vapor. Their reactivity decreases due to the increasing size of their atoms, making it harder to attract an additional electron. Astatine, like francium and radium, is radioactive and extremely rare.
The Noble Gases (Group 18): Relatively Unreactive
The noble gases (helium, neon, argon, krypton, xenon, and radon) in Group 18 are remarkably unreactive. They have a full valence shell of electrons (eight electrons, except for helium which has two), making them exceptionally stable. This stable configuration means they have little tendency to gain, lose, or share electrons, resulting in their low reactivity. However, under specific conditions, some heavier noble gases (like xenon and krypton) can form compounds with highly electronegative elements like fluorine and oxygen.
Factors Affecting Reactivity
Several factors influence an element's reactivity:
-
Electronic Configuration: The number of valence electrons and how readily they are gained, lost, or shared directly impacts reactivity. Elements with nearly full or empty valence shells tend to be more reactive than those with half-full shells.
-
Electronegativity: This measures an atom's ability to attract electrons in a chemical bond. Highly electronegative elements readily attract electrons, making them reactive.
-
Ionization Energy: The energy required to remove an electron from an atom. Elements with low ionization energy easily lose electrons, thus exhibiting high reactivity.
-
Atomic Size: Larger atoms generally have lower ionization energies and electronegativities, leading to higher reactivity (for metals) and lower reactivity (for non-metals).
Safety Precautions
Handling highly reactive elements requires meticulous safety measures:
-
Inert Atmosphere: Many reactive elements must be handled in an inert atmosphere (e.g., argon or nitrogen) to prevent reactions with air and moisture.
-
Specialized Equipment: Special containers and equipment are needed to prevent accidental exposure and reactions.
-
Personal Protective Equipment (PPE): Appropriate PPE, including gloves, goggles, and lab coats, is essential to protect against chemical burns and other hazards.
-
Controlled Environment: Experiments involving highly reactive elements should be conducted in a well-ventilated area or a fume hood to minimize exposure to hazardous gases.
-
Proper Training: Individuals handling reactive elements must receive thorough training on safe handling procedures and emergency response protocols.
Conclusion
The periodic table offers a fascinating insight into the chemical behavior of elements. The most reactive elements, such as the alkali metals and halogens, demonstrate striking reactions due to their electronic configurations and inherent properties. Understanding their reactivity is crucial for various applications, from industrial processes to medical advancements. However, handling these elements requires utmost caution and adherence to strict safety protocols to prevent accidents and ensure the safety of personnel. The study of these elements continues to expand our understanding of chemical interactions and opens new avenues for scientific exploration.
Latest Posts
Latest Posts
-
A Chemical Bond Is Formed When Electrons Are
Mar 25, 2025
-
Memory That Is Active At Any Given Moment Is Called
Mar 25, 2025
-
Domain And Range In Quadratic Functions
Mar 25, 2025
-
How Does Ice Contribute To Erosion
Mar 25, 2025
-
Amino Acid Trivia Based On Acidity
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Most Reactive Elements On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
