A Chemical Bond Is Formed When Electrons Are
Muz Play
Mar 25, 2025 · 6 min read
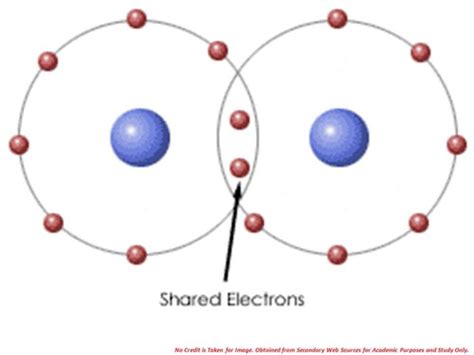
Table of Contents
A Chemical Bond is Formed When Electrons Are…Shared, Transferred, or Otherwise Interacted With!
Chemical bonds are the fundamental forces that hold atoms together to form molecules and compounds. Understanding how these bonds form is crucial to grasping the properties of matter and the behavior of chemical reactions. The simple answer to the question, "A chemical bond is formed when electrons are...?" is: A chemical bond is formed when electrons are shared, transferred, or otherwise significantly interacted with between atoms. Let's delve deeper into the nuances of this statement.
The Driving Force: Achieving Stability
Atoms are inherently driven towards stability. This stability is typically achieved by having a full outer electron shell (also known as the valence shell). Atoms with incomplete valence shells readily interact with other atoms to gain, lose, or share electrons and reach this stable configuration. This drive for stability is the fundamental principle underlying the formation of chemical bonds.
Types of Chemical Bonds: A Closer Look
Several types of chemical bonds exist, each characterized by how electrons are involved:
1. Ionic Bonds: The Transfer of Electrons
Ionic bonds form through the transfer of electrons from one atom to another. This transfer creates ions: atoms with a net electrical charge. One atom loses electrons and becomes a positively charged cation, while the other atom gains these electrons and becomes a negatively charged anion. The electrostatic attraction between these oppositely charged ions forms the ionic bond.
Characteristics of Ionic Bonds:
- High melting and boiling points: The strong electrostatic forces require significant energy to overcome.
- Crystalline structure: Ions are arranged in a regular, repeating pattern in a lattice structure.
- Good conductors of electricity when dissolved or molten: The mobile ions can carry an electric current.
- Brittle: Displacement of ions can lead to repulsion and fracture.
Example: Sodium chloride (NaCl), common table salt. Sodium (Na) readily loses one electron to achieve a stable configuration, becoming Na⁺. Chlorine (Cl) readily gains one electron to achieve a stable configuration, becoming Cl⁻. The electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond in NaCl.
2. Covalent Bonds: The Sharing of Electrons
Covalent bonds form through the sharing of electrons between atoms. This shared electron pair is attracted to the nuclei of both atoms, holding them together. The shared electrons effectively fill the valence shells of both atoms, leading to stability.
Characteristics of Covalent Bonds:
- Lower melting and boiling points than ionic bonds (generally): The forces holding the molecules together are weaker than the electrostatic forces in ionic bonds.
- Can be solids, liquids, or gases at room temperature: This depends on the strength of intermolecular forces.
- Generally poor conductors of electricity: There are no freely moving charges.
- Can be highly diverse in structure and properties: Depending on the atoms and the number of shared electrons, various molecules with different properties can be formed.
Example: Water (H₂O). Each hydrogen atom shares one electron with the oxygen atom, and the oxygen atom shares one electron with each hydrogen atom. This sharing creates two covalent bonds, forming a stable water molecule. The shared electrons are attracted to both the hydrogen and oxygen nuclei, holding the molecule together. Another example is methane (CH₄) where carbon shares one electron with each of four hydrogen atoms.
Types of Covalent Bonds:
- Single Covalent Bond: One pair of electrons is shared between two atoms.
- Double Covalent Bond: Two pairs of electrons are shared between two atoms.
- Triple Covalent Bond: Three pairs of electrons are shared between two atoms.
The number of bonds an atom can form depends on the number of electrons it needs to complete its valence shell. This is often determined by the group number of the element in the periodic table.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals. In this type of bond, valence electrons are delocalized, meaning they are not associated with any particular atom but rather move freely throughout the metal lattice. This creates a "sea" of electrons surrounding positively charged metal ions. The electrostatic attraction between the positive ions and the sea of electrons holds the metal together.
Characteristics of Metallic Bonds:
- High melting and boiling points (generally): The strong attraction between the positive ions and the sea of electrons requires significant energy to overcome.
- Good conductors of electricity and heat: The free-moving electrons can easily carry electric current and transfer thermal energy.
- Malleable and ductile: The delocalized electrons allow the metal ions to slide past each other without disrupting the metallic bond.
- Shiny (lustrous): The free electrons interact with light, leading to a characteristic metallic sheen.
Example: Copper (Cu). Copper atoms contribute their valence electrons to the sea of electrons, allowing for excellent electrical conductivity and the characteristic malleability of the metal.
4. Hydrogen Bonds: Special Intermolecular Forces
Hydrogen bonds are a special type of intermolecular force, meaning they occur between molecules rather than within them. They involve a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and another electronegative atom in a separate molecule. The hydrogen atom's slightly positive charge is attracted to the electronegative atom's slightly negative charge, creating a relatively weak bond compared to ionic and covalent bonds.
Characteristics of Hydrogen Bonds:
- Weaker than ionic and covalent bonds: They are intermolecular forces, not intramolecular forces.
- Crucial for many biological processes: They are responsible for the properties of water, the structure of proteins and DNA, and many other biological functions.
- Influence boiling and melting points: Hydrogen bonds increase the boiling and melting points of substances.
Example: Water (H₂O). The slightly positive hydrogen atoms in one water molecule are attracted to the slightly negative oxygen atoms in other water molecules, forming hydrogen bonds. These bonds contribute to the high boiling point and surface tension of water.
Factors Influencing Bond Strength
Several factors influence the strength of a chemical bond:
- Electronegativity: The ability of an atom to attract electrons in a covalent bond. A large difference in electronegativity between atoms leads to a polar covalent bond, where the electrons are shared unequally. A very large difference leads to an ionic bond.
- Bond length: The distance between the nuclei of two bonded atoms. Shorter bond lengths generally indicate stronger bonds.
- Bond order: The number of electron pairs shared between two atoms. Higher bond orders generally indicate stronger bonds.
- Number of electrons involved: More electrons shared or transferred generally results in stronger bonds.
Beyond the Basics: More Complex Interactions
While the above categories cover the primary types of chemical bonds, the reality is often more nuanced. Many molecules exhibit characteristics of more than one type of bonding. For example, some molecules may have both ionic and covalent character (polar covalent bonds). Others can display complex interactions such as coordinate covalent bonds (also known as dative covalent bonds) where both electrons in a bonding pair originate from one atom. Furthermore, intermolecular forces like van der Waals forces, while weaker than the primary bond types, significantly influence the physical properties of substances.
Conclusion: The Electron's Central Role
In essence, chemical bonds are all about electrons. The way electrons are shared, transferred, or otherwise interacted with between atoms dictates the type of bond formed, and consequently the properties of the resulting substance. Understanding this fundamental principle is key to understanding the structure and behavior of matter in the world around us, from the simplest molecules to the most complex biological systems. The electron's role in establishing these interactions is absolutely paramount in the field of chemistry. This multifaceted understanding provides a complete picture of the dynamic world of chemical bonding and underscores the profound influence of electron configuration on the properties of matter.
Latest Posts
Latest Posts
-
What Structure Is Most Important In Forming The Tetrads
Mar 26, 2025
-
What Color Is An Animal Cell
Mar 26, 2025
-
Differentiation And Integration Of Power Series
Mar 26, 2025
-
Do Fingernails And Hair Have Chitin In Them
Mar 26, 2025
-
Separation Of Variables Partial Differential Equations Examples
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about A Chemical Bond Is Formed When Electrons Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
