Calculate The Ph At The Equivalence Point
Muz Play
Mar 22, 2025 · 6 min read
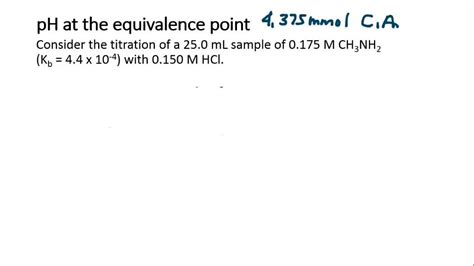
Table of Contents
Calculating the pH at the Equivalence Point of a Titration
Determining the pH at the equivalence point of a titration is crucial for understanding the stoichiometry of a reaction and selecting appropriate indicators. The equivalence point signifies the point in a titration where the moles of titrant added exactly react with the moles of analyte present. However, the pH at this point isn't always 7, as commonly misconceived. The actual pH depends heavily on the nature of the acid and base involved in the titration. This article will explore the different scenarios and the methods for calculating the pH at the equivalence point.
Types of Titrations and Their Equivalence Point pH
Before delving into the calculations, it's essential to classify the different types of titrations:
1. Strong Acid-Strong Base Titration
This is the simplest case. When a strong acid (e.g., HCl) is titrated with a strong base (e.g., NaOH), the equivalence point pH is always 7. This is because the reaction produces water and a neutral salt. The conjugate acid and base of a strong acid and base are extremely weak and do not affect the pH significantly.
Example: The titration of 0.1 M HCl with 0.1 M NaOH. At the equivalence point, the solution contains only NaCl and water. NaCl is a neutral salt, hence the pH remains 7.
2. Weak Acid-Strong Base Titration
In this scenario, the pH at the equivalence point is always greater than 7 (basic). The reason is the hydrolysis of the conjugate base of the weak acid. The conjugate base reacts with water, producing hydroxide ions (OH⁻) and increasing the pH.
Understanding Hydrolysis: When the salt of a weak acid and strong base dissolves in water, the conjugate base accepts a proton from water, forming hydroxide ions and the weak acid. The extent of this hydrolysis depends on the strength of the weak acid. A weaker acid will have a stronger conjugate base, leading to greater hydrolysis and a higher pH at the equivalence point.
Calculation: To calculate the pH, we need to consider the equilibrium of the conjugate base with water. This involves using the Kb (base dissociation constant) of the conjugate base, which is related to the Ka (acid dissociation constant) of the weak acid by the equation:
Kw = Ka * Kb (where Kw is the ion product constant of water, 1.0 x 10⁻¹⁴ at 25°C)
The pH can be calculated using the following steps:
-
Determine the concentration of the conjugate base: This is equal to the initial concentration of the weak acid divided by the volume at the equivalence point.
-
Use the Kb to calculate the hydroxide ion concentration [OH⁻]: This usually requires solving a quadratic equation, though approximations can sometimes be made if Kb is very small.
-
Calculate the pOH: pOH = -log₁₀[OH⁻]
-
Calculate the pH: pH = 14 - pOH
3. Strong Acid-Weak Base Titration
Conversely, when a strong acid is titrated with a weak base, the pH at the equivalence point is always less than 7 (acidic). This is due to the hydrolysis of the conjugate acid of the weak base. The conjugate acid donates a proton to water, producing hydronium ions (H₃O⁺) and lowering the pH.
Calculation: Similar to the weak acid-strong base titration, we need to use the Ka of the conjugate acid (related to the Kb of the weak base) to calculate the hydronium ion concentration and subsequently the pH.
4. Weak Acid-Weak Base Titration
This case is the most complex. The pH at the equivalence point depends on the relative strengths of the weak acid and weak base. It might be acidic, basic, or even close to neutral depending on which conjugate is stronger. A precise calculation requires considering both the Ka of the weak acid and the Kb of the weak base and involves solving more complex equilibrium expressions.
Detailed Calculation Examples
Let's illustrate the calculations with specific examples:
Example 1: Weak Acid-Strong Base Titration
Consider the titration of 25.0 mL of 0.10 M acetic acid (CH₃COOH, Ka = 1.8 x 10⁻⁵) with 0.10 M NaOH.
-
Equivalence Point: At the equivalence point, the moles of NaOH added equal the moles of CH₃COOH initially present:
Moles CH₃COOH = (0.10 mol/L) * (0.025 L) = 0.0025 mol
Volume NaOH at equivalence point = 0.0025 mol / (0.10 mol/L) = 0.025 L = 25.0 mL
Total volume at equivalence point = 25.0 mL + 25.0 mL = 50.0 mL
-
Concentration of Acetate Ion (CH₃COO⁻):
[CH₃COO⁻] = 0.0025 mol / 0.050 L = 0.050 M
-
Kb of Acetate Ion:
Kb = Kw / Ka = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) = 5.6 x 10⁻¹⁰
-
Hydroxide Ion Concentration [OH⁻]: (using an approximation since Kb is small)
Kb = [OH⁻]² / [CH₃COO⁻]
[OH⁻] = √(Kb * [CH₃COO⁻]) = √(5.6 x 10⁻¹⁰ * 0.050) ≈ 5.3 x 10⁻⁶ M
-
pOH and pH:
pOH = -log₁₀(5.3 x 10⁻⁶) ≈ 5.28
pH = 14 - pOH ≈ 8.72
Example 2: Strong Acid-Weak Base Titration
Let's consider the titration of 20.0 mL of 0.20 M HCl with 0.10 M NH₃ (Kb = 1.8 x 10⁻⁵).
-
Equivalence Point: Moles HCl = (0.20 mol/L) * (0.020 L) = 0.0040 mol
Volume NH₃ at equivalence point = 0.0040 mol / (0.10 mol/L) = 0.040 L = 40.0 mL
Total volume at equivalence point = 20.0 mL + 40.0 mL = 60.0 mL
-
Concentration of Ammonium Ion (NH₄⁺):
[NH₄⁺] = 0.0040 mol / 0.060 L ≈ 0.067 M
-
Ka of Ammonium Ion:
Ka = Kw / Kb = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) ≈ 5.6 x 10⁻¹⁰
-
Hydronium Ion Concentration [H₃O⁺]: (approximation due to small Ka)
Ka = [H₃O⁺]² / [NH₄⁺]
[H₃O⁺] = √(Ka * [NH₄⁺]) = √(5.6 x 10⁻¹⁰ * 0.067) ≈ 6.1 x 10⁻⁶ M
-
pH:
pH = -log₁₀(6.1 x 10⁻⁶) ≈ 5.21
Factors Affecting pH at the Equivalence Point
Several factors influence the pH at the equivalence point, including:
-
Concentration of the acid and base: Higher concentrations generally lead to less deviation from the expected pH (7 for strong acid-strong base, >7 for weak acid-strong base, <7 for strong acid-weak base).
-
Temperature: The Kw value changes with temperature, affecting the pH calculations.
-
Ionic strength: The presence of other ions in the solution can affect the activity coefficients of the ions involved in the equilibrium, impacting the pH. This effect is often neglected in simpler calculations.
-
Presence of other acids or bases: Any impurities or buffer components present in the solution will alter the pH.
Importance of pH at the Equivalence Point
The pH at the equivalence point is critical for several reasons:
-
Indicator Selection: The appropriate indicator for a titration must change color around the equivalence point pH. Choosing an incorrect indicator can lead to inaccurate results.
-
Understanding Reaction Stoichiometry: The pH at the equivalence point provides insights into the strength of the acid and base involved.
-
Analytical Applications: Titrations are used in numerous analytical procedures to determine the concentration of unknown substances. Accurate determination of the equivalence point pH is vital for these applications.
-
Environmental monitoring: Determining pH is crucial in many environmental contexts, such as monitoring water quality and soil acidity, where titrations are commonly employed.
This comprehensive guide provides a detailed understanding of calculating the pH at the equivalence point for various titration types. Remember that accurate calculations often require considering the appropriate equilibrium expressions and potential approximations, depending on the relative strengths of the acid and base involved. Understanding these principles is crucial for anyone working in analytical chemistry, environmental science, or any field requiring precise pH measurements.
Latest Posts
Latest Posts
-
What Remains Constant In Charles Law
Mar 23, 2025
-
How To Diagonalize A 2x2 Matrix
Mar 23, 2025
-
Solve Radical Equations With Extraneous Solutions
Mar 23, 2025
-
Similarities Between Plant And Animal Cells
Mar 23, 2025
-
Label The Directional Terms Based On The Arrows
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
