What Remains Constant In Charles Law
Muz Play
Mar 23, 2025 · 6 min read
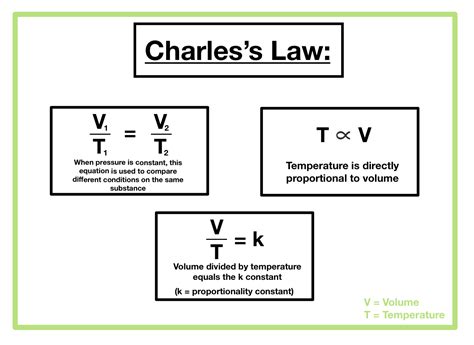
Table of Contents
What Remains Constant in Charles's Law: A Deep Dive into Volume-Temperature Relationships
Charles's Law, a cornerstone of ideal gas behavior, describes the direct proportionality between the volume and temperature of a gas, provided the pressure and the amount of gas remain constant. Understanding what remains constant – and equally importantly, what doesn't – is crucial to accurately applying and interpreting this fundamental gas law. This in-depth exploration will delve into the intricacies of Charles's Law, clarifying its conditions, exploring its limitations, and illustrating its practical applications.
The Invariable Parameters: Pressure and Amount of Gas
The bedrock of Charles's Law is the unwavering constancy of pressure and the amount of gas (number of moles). Let's examine each in detail:
Constant Pressure: The Unchanging Force
Maintaining constant pressure is paramount. This means the external pressure exerted on the gas remains unchanged throughout the experiment or observation. Any change in pressure will invalidate the direct proportionality predicted by Charles's Law. Imagine a gas confined within a flexible container. If the external pressure increases, the gas volume will decrease, even if the temperature rises. This deviation occurs because the increased pressure counteracts the temperature-induced volume expansion. Therefore, to observe the pure effect of temperature on volume as described by Charles's Law, the pressure must be held consistently.
Techniques to ensure constant pressure vary depending on the experimental setup. In simpler scenarios, an open container at atmospheric pressure can suffice, provided atmospheric pressure itself remains relatively stable. For more controlled experiments, specialized equipment such as barostats or isobaric systems are employed to maintain precise pressure levels.
Constant Amount of Gas: The Fixed Number of Molecules
Equally crucial is maintaining a constant amount of gas. This refers to the number of gas molecules (or moles) present within the system. No gas should be added or removed throughout the observation period. If the amount of gas changes, the relationship between volume and temperature will no longer conform to Charles's Law. For example, if the gas leaks out of the container, the volume will decrease regardless of the temperature change. Similarly, if more gas is introduced, the volume will increase, irrespective of the temperature.
Ensuring a constant amount of gas often involves using a sealed, airtight container. Any leaks or gas exchange with the surrounding environment must be meticulously avoided. In advanced experiments, precise mass measurements before and after the experiment can confirm the constancy of the gas's mass and, therefore, its amount.
The Variables: Volume and Temperature
While pressure and amount of gas are held constant, the volume and temperature of the gas are the variables of interest in Charles's Law. Their relationship forms the very essence of the law.
Volume: The Expanding Space
The volume (V) of the gas refers to the space occupied by the gas molecules. This volume is directly proportional to the absolute temperature (T) of the gas under constant pressure and amount. As temperature increases, the kinetic energy of the gas molecules increases, leading to more frequent and forceful collisions with the container walls. This, in turn, causes the gas to expand, thus increasing its volume. Conversely, lowering the temperature decreases the kinetic energy, reducing the volume.
Accurate volume measurement is vital for applying Charles's Law. Units such as liters (L), cubic meters (m³), or milliliters (mL) are commonly used. The choice of unit depends on the scale of the experiment.
Temperature: The Driving Force
The temperature (T) used in Charles's Law is always the absolute temperature, measured in Kelvin (K). Using Celsius or Fahrenheit will lead to incorrect results because these scales have arbitrary zero points. The absolute temperature scale (Kelvin) starts at absolute zero (-273.15°C), representing the theoretical point where all molecular motion ceases.
This is a crucial point of understanding. The direct proportionality exists between volume and absolute temperature, not the relative temperature measured on Celsius or Fahrenheit scales. Converting Celsius to Kelvin is a straightforward process: K = °C + 273.15.
The Mathematical Expression: A Concise Representation
Charles's Law can be succinctly expressed mathematically as:
V₁/T₁ = V₂/T₂
Where:
- V₁ is the initial volume
- T₁ is the initial absolute temperature
- V₂ is the final volume
- T₂ is the final absolute temperature
This equation highlights the direct proportionality between volume and absolute temperature. If the temperature doubles (while pressure and amount of gas remain constant), the volume will also double.
Limitations and Deviations: When Charles's Law Fails
While Charles's Law provides a valuable framework for understanding gas behavior, it's crucial to acknowledge its limitations. It applies most accurately to ideal gases – a theoretical concept that assumes gas molecules have negligible volume and exert no intermolecular forces. Real gases, however, deviate from ideal behavior, especially at high pressures and low temperatures.
Under these conditions, the attractive forces between gas molecules become significant, causing the gas to deviate from the predictions of Charles's Law. At high pressures, the volume occupied by the gas molecules themselves becomes a non-negligible fraction of the total volume. These deviations are often accounted for using more complex equations of state, such as the van der Waals equation.
Applications: From Balloons to Breath
Charles's Law finds numerous practical applications across diverse fields:
Meteorology: Weather Balloons and Forecasting
Weather balloons rely on Charles's Law. As the balloon ascends, the atmospheric pressure decreases. Consequently, the gas inside the balloon expands, increasing its volume. Accurate predictions of this expansion are crucial for accurate weather data collection.
Automotive Engineering: Tire Pressure and Temperature
The temperature of tires affects their pressure. On a hot day, tire pressure increases, potentially leading to blowouts if not properly monitored. Understanding Charles's Law helps in maintaining appropriate tire pressure under varying temperature conditions.
Medical Applications: Respiratory Function
Charles's Law plays a role in understanding respiratory function. The process of inhalation and exhalation involves changes in lung volume and temperature, directly influencing the gas pressure within the lungs.
Everyday Applications: Hot Air Balloons
The principle behind hot air balloons is a direct application of Charles's Law. Heating the air inside the balloon reduces its density and increases its volume, causing the balloon to rise. Controlling the temperature of the air dictates the balloon's altitude.
Conclusion: A Fundamental Principle with Broad Reach
Charles's Law, with its emphasis on the constancy of pressure and amount of gas, offers a fundamental understanding of the relationship between the volume and temperature of a gas. While its ideal gas assumptions lead to limitations under extreme conditions, its applicability in numerous real-world scenarios remains substantial. From meteorology to medicine, understanding and applying Charles's Law continues to be crucial in many scientific and engineering endeavors. Remembering the importance of maintaining constant pressure and amount of gas while focusing on the direct proportionality between absolute temperature and volume allows for successful application of this cornerstone principle of gas behavior. Precise measurements and an understanding of its limitations ensure accurate predictions and applications of Charles's Law in a variety of fields.
Latest Posts
Latest Posts
-
Metals Are Located Where On The Periodic Table
Mar 25, 2025
-
All Organisms Are Composed Of One Or More Cells
Mar 25, 2025
-
Anything That Interferes With Successful Communication Is Said To Be
Mar 25, 2025
-
Construct The Confidence Interval For The Population Mean M
Mar 25, 2025
-
Is Mixing A Chemical Or Physical Change
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Remains Constant In Charles Law . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
