Metals Are Located Where On The Periodic Table
Muz Play
Mar 25, 2025 · 5 min read
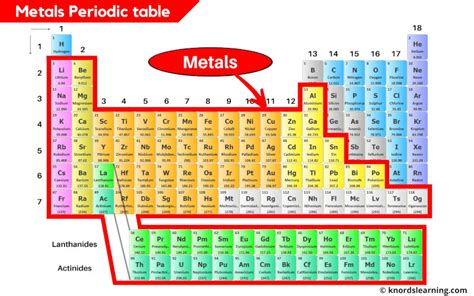
Table of Contents
Metals: Their Location and Properties on the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. One of the most fundamental classifications within the table is the distinction between metals, nonmetals, and metalloids. Understanding where metals are located on the periodic table is crucial for grasping their characteristic properties and predicting their behavior in various chemical reactions. This comprehensive guide will delve deep into the location of metals, their defining features, and how their position on the table influences their properties.
The Broad Location of Metals on the Periodic Table
Metals overwhelmingly dominate the periodic table. They occupy the left-hand side and the middle of the table, forming a vast majority of the known elements. A clear diagonal line separates metals from nonmetals, running roughly from Boron (B) to Astatine (At). Elements to the left of this line are generally considered metals, while those to the right are nonmetals. Elements directly adjacent to this line exhibit properties of both metals and nonmetals, and are classified as metalloids (or semimetals).
Key Regions Where Metals Are Found:
-
Alkali Metals (Group 1): Located in the first column, these are highly reactive metals, readily losing one electron to form +1 ions. Their reactivity increases down the group.
-
Alkaline Earth Metals (Group 2): Found in the second column, these metals are also reactive, but less so than the alkali metals. They typically lose two electrons to form +2 ions.
-
Transition Metals (Groups 3-12): This large block in the middle of the table comprises metals with variable oxidation states, leading to a wide range of chemical behaviors and compound formation. Many are known for their colorful compounds and catalytic properties.
-
Inner Transition Metals (Lanthanides and Actinides): These two rows at the bottom of the table represent elements with unique electronic configurations and properties. The lanthanides (rare earth elements) are relatively similar in chemical behavior, while the actinides are largely radioactive.
-
Post-Transition Metals: These metals are located towards the right of the transition metals, often exhibiting weaker metallic properties compared to their counterparts to the left. They include elements like Aluminum (Al), Tin (Sn), and Lead (Pb).
Properties of Metals: A Consequence of their Atomic Structure
The location of metals on the periodic table directly relates to their fundamental properties. These properties stem from the characteristic arrangement of electrons in their atoms.
Key Metallic Properties:
-
Electrical Conductivity: Metals are excellent conductors of electricity. This is because their valence electrons are loosely held and can move freely throughout the metallic lattice, forming a "sea" of delocalized electrons. This allows for the easy flow of electric current.
-
Thermal Conductivity: Metals efficiently conduct heat. The freely moving electrons can readily transfer kinetic energy, resulting in rapid heat transfer throughout the material.
-
Malleability and Ductility: Metals are easily shaped (malleable) and drawn into wires (ductile). This is due to the ability of metal atoms to slide past each other in the metallic lattice without disrupting the overall structure.
-
Luster: Most metals possess a characteristic metallic luster—a shiny appearance. This is due to the interaction of light with the delocalized electrons in the metal.
-
Hardness and Strength: While some metals are soft (like sodium), many are relatively hard and strong, making them suitable for structural applications. The strength varies significantly depending on the specific metal and its crystalline structure.
-
Density: Metals generally have relatively high densities compared to nonmetals. This is because of their closely packed atomic structures.
-
Melting and Boiling Points: The melting and boiling points of metals vary widely, depending on factors such as the number of valence electrons and the strength of metallic bonding. Transition metals, for example, tend to have high melting and boiling points due to strong metallic bonds.
Exceptions and Gray Areas: Metalloids and Unusual Metallic Behavior
While the general location of metals on the periodic table is straightforward, there are some exceptions and gray areas. These stem from the gradual transition in properties across the periodic table.
Metalloids (Semimetals):
Elements located along the diagonal line separating metals and nonmetals exhibit properties of both metals and nonmetals, making them metalloids. These include Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), and Polonium (Po). Their conductivity can vary depending on factors like temperature and applied pressure. They are often semiconductors, making them crucial in electronics.
Unusual Metallic Behavior:
Some metals might show variations in their typical metallic properties. For instance, Mercury (Hg) is a liquid at room temperature, a unique property among metals. Certain transition metals, like Zinc (Zn) and Cadmium (Cd), can exhibit somewhat weaker metallic bonding and properties compared to other transition metals.
The Periodic Table and Predicting Metallic Properties: Trends and Patterns
The periodic table allows us to predict certain trends in metallic properties. These trends are generally observed within groups (vertical columns) and periods (horizontal rows).
Trends within Groups:
-
Alkali Metals (Group 1): Reactivity generally increases down the group due to increasing atomic size and decreasing ionization energy.
-
Alkaline Earth Metals (Group 2): Similar to alkali metals, reactivity increases down the group.
-
Transition Metals: Trends within transition metal groups are less straightforward due to complex electronic configurations and variable oxidation states.
Trends within Periods:
- Generally, metallic character decreases from left to right across a period, as the effective nuclear charge increases, drawing electrons closer to the nucleus and reducing their availability for metallic bonding.
Conclusion: The Periodic Table as a Predictive Tool for Metallic Properties
The location of metals on the periodic table is a fundamental concept in chemistry. Their position directly correlates with their characteristic properties, such as electrical conductivity, malleability, and ductility. By understanding the arrangement of elements, we can predict the behavior of metals and their interactions with other elements. While some exceptions and variations exist, the periodic table serves as a powerful tool for understanding and predicting the properties of metals. This knowledge is crucial for various applications across numerous fields, including materials science, engineering, and electronics. Further exploration of specific groups and periods within the table will unveil more intricate details about the remarkable diversity of metals and their unique contributions to our world.
Latest Posts
Latest Posts
-
What Is The Difference Between Gene Mutations And Chromosomal Mutations
Mar 26, 2025
-
What Is A Positively Charged Subatomic Particle
Mar 26, 2025
-
The Is The Fundamental Unit Of Life
Mar 26, 2025
-
How Many Phosphates Does Adp Have
Mar 26, 2025
-
What Is An Equation Of A Horizontal Line
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Metals Are Located Where On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
