What Is A Positively Charged Subatomic Particle
Muz Play
Mar 26, 2025 · 7 min read
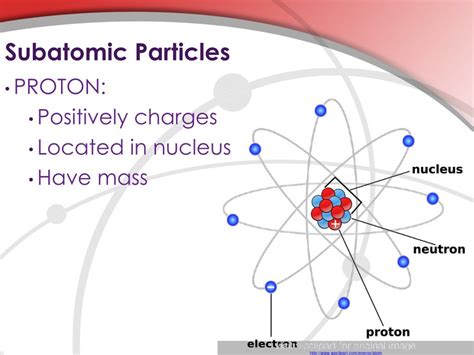
Table of Contents
What is a Positively Charged Subatomic Particle? A Deep Dive into Protons
The universe, at its most fundamental level, is built from tiny particles. Understanding these particles is key to understanding everything around us, from the smallest atom to the largest galaxy. One crucial piece of this puzzle is the positively charged subatomic particle, the proton. This article delves deep into the nature of protons, exploring their properties, discovery, role in atomic structure, and their significance in physics and beyond.
Understanding Subatomic Particles: A Brief Overview
Before diving into the specifics of protons, let's briefly revisit the concept of subatomic particles. Atoms, the basic building blocks of matter, are not indivisible as once thought. Instead, they consist of even smaller components:
- Protons: These carry a positive electrical charge.
- Neutrons: These carry no electrical charge (they are neutral).
- Electrons: These carry a negative electrical charge.
Protons and neutrons reside within the atom's nucleus, a dense central region, while electrons orbit this nucleus in electron shells. The arrangement and number of these subatomic particles determine the properties of an element.
The Proton: The Positively Charged Core
The proton, denoted by the symbol p or p⁺, is a fundamental component of atomic nuclei. It's significantly more massive than an electron, possessing approximately 1836 times the electron's mass. This significant mass difference contributes greatly to the overall mass of an atom, with the nucleus containing almost all of the atom's mass. Crucially, a proton carries a single positive elementary charge, equal in magnitude but opposite in sign to the charge of an electron.
Key Properties of Protons:
- Charge: +1 elementary charge (approximately 1.602 x 10⁻¹⁹ Coulombs)
- Mass: Approximately 1.673 x 10⁻²⁷ kilograms (or 1 atomic mass unit, amu)
- Spin: ½ (a fundamental quantum property related to angular momentum)
- Composition: Protons are composite particles, meaning they are made up of smaller constituents called quarks. Specifically, they consist of two up quarks and one down quark.
- Stability: Protons are remarkably stable particles. While theoretically they could decay, the half-life is so extraordinarily long (estimated to be at least 10³⁴ years) that they are considered practically stable for all practical purposes.
The Discovery of the Proton: Unraveling the Atomic Structure
The journey to understanding the proton was a gradual process, building upon decades of scientific investigation. While the concept of an atom had been proposed centuries earlier by Greek philosophers, the understanding of its internal structure emerged much later.
The discovery of the proton is often linked to Ernest Rutherford's gold foil experiment in 1911. This experiment involved bombarding a thin gold foil with alpha particles (positively charged helium nuclei). While most of the alpha particles passed through the foil undeflected, a small percentage were deflected at large angles, some even bouncing back. This unexpected result led Rutherford to propose the existence of a dense, positively charged nucleus at the center of the atom.
Subsequent experiments refined the understanding of the nucleus. In 1919, Rutherford himself conducted experiments that led to the discovery of the proton. By bombarding nitrogen gas with alpha particles, he observed the emission of protons, providing direct evidence for their existence within the atomic nucleus. This landmark discovery marked a pivotal moment in the understanding of atomic structure.
Protons and Atomic Number: Defining Elements
The number of protons in an atom's nucleus determines its atomic number and, consequently, its identity as a specific chemical element. For example, hydrogen (H) has one proton, helium (He) has two, lithium (Li) has three, and so on. This fundamental relationship between protons and atomic number is a cornerstone of the periodic table, which organizes elements based on their atomic number and chemical properties.
Isotopes, variants of the same element with different numbers of neutrons, possess the same number of protons but vary in mass. For example, carbon-12 (¹²C) and carbon-14 (¹⁴C) are isotopes of carbon, both having six protons, but differing in the number of neutrons (six and eight, respectively).
Quarks: The Building Blocks of Protons
Protons, as previously mentioned, are not fundamental particles; they are composed of smaller, more fundamental particles called quarks. The Standard Model of particle physics describes six types (or "flavors") of quarks: up, down, charm, strange, top, and bottom. Each quark carries a fractional electric charge.
A proton is composed of three quarks: two up quarks (each with a charge of +⅔) and one down quark (with a charge of -⅓). The combination of these charges results in the proton's overall positive charge of +1. The strong nuclear force, mediated by gluons, holds these quarks together within the proton.
The intricate interactions between quarks and gluons within the proton are described by Quantum Chromodynamics (QCD), a complex theory that forms a part of the Standard Model. Understanding these interactions is crucial for a deeper understanding of the proton's properties and behavior.
Protons in Nuclear Reactions: Fusion and Fission
Protons play a vital role in nuclear reactions, the processes that involve changes in the nuclei of atoms. Two significant examples are nuclear fusion and nuclear fission.
Nuclear Fusion: This process involves the combining of lighter atomic nuclei (such as hydrogen isotopes) to form heavier nuclei, releasing enormous amounts of energy. The proton, being a fundamental component of hydrogen nuclei, plays a central role in fusion reactions that power stars, including our own sun. The fusion of hydrogen isotopes in the sun converts protons into helium, releasing immense energy in the process.
Nuclear Fission: This process involves the splitting of a heavy atomic nucleus (such as uranium or plutonium) into smaller nuclei, again releasing a substantial amount of energy. While protons are part of the heavier nuclei undergoing fission, the process primarily focuses on the manipulation of nuclear forces and the instability of heavy nuclei.
Protons in Particle Accelerators: Exploring the Subatomic World
Particle accelerators, such as the Large Hadron Collider (LHC), are powerful tools used to accelerate protons (and other particles) to incredibly high speeds, allowing scientists to study their properties and interactions at high energies. By colliding protons at such high speeds, researchers can create new particles and investigate fundamental forces of nature. These experiments have provided profound insights into the Standard Model of particle physics and continue to push the boundaries of our understanding of the universe.
The Proton's Significance in Science and Technology
The proton's significance extends beyond fundamental physics. Its properties and behavior have far-reaching implications in various scientific and technological fields:
- Nuclear Medicine: Proton therapy utilizes beams of protons to target and destroy cancerous tumors, minimizing damage to surrounding healthy tissues. This technique offers a more precise and less invasive form of cancer treatment compared to conventional radiation therapy.
- Materials Science: Understanding the behavior of protons is crucial in designing new materials with specific properties. Protons’ role in chemical bonding and reactions is fundamental to many materials science applications.
- Nuclear Power: Both nuclear fusion and fission, reliant on protons, offer potential solutions to global energy demands, although each carries its own challenges and risks.
Future Research: Unanswered Questions about Protons
Despite our extensive knowledge of protons, several aspects remain subjects of ongoing research:
- Proton Spin Structure: The precise contribution of quarks and gluons to the proton's overall spin is still an area of active investigation.
- Proton Size and Shape: The exact size and shape of the proton are subject to ongoing refinement.
- Proton Decay: While extremely unlikely, the possibility of proton decay remains a topic of research, with implications for theories beyond the Standard Model.
Conclusion: A Cornerstone of Matter
The positively charged subatomic particle, the proton, stands as a cornerstone of matter and our understanding of the universe. From defining the elements on the periodic table to powering stars and enabling cutting-edge technologies, protons play a vital role across diverse scientific and technological domains. Further research continues to unveil their deeper mysteries, pushing the boundaries of our knowledge and paving the way for future discoveries. The journey to fully understanding the proton, this tiny yet mighty particle, remains an ongoing adventure in scientific exploration.
Latest Posts
Latest Posts
-
Half Life Sample Problems With Answers
Mar 29, 2025
-
Does Carbon Follow The Octet Rule
Mar 29, 2025
-
The Substance That Is Being Dissolved By A Solvent
Mar 29, 2025
-
How To Factor Trinomials When A Is Not 1
Mar 29, 2025
-
How To Determine A Strong Acid
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is A Positively Charged Subatomic Particle . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
