The Substance That Is Being Dissolved By A Solvent.
Muz Play
Mar 30, 2025 · 7 min read
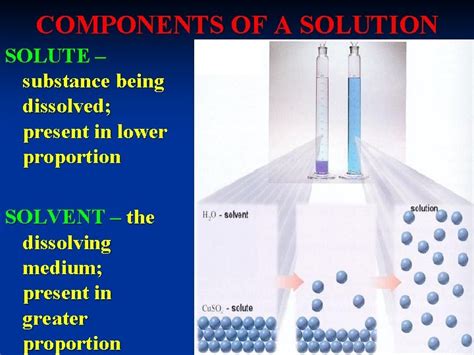
Table of Contents
The Solute: Understanding the Dissolved Substance
The world around us is a testament to the power of solutions. From the air we breathe to the blood flowing through our veins, solutions – homogenous mixtures of two or more substances – are ubiquitous. A key component of any solution is the solute, the substance that is dissolved within another substance, known as the solvent. This comprehensive article delves into the fascinating world of solutes, exploring their properties, behaviours, and importance across various scientific fields and everyday applications.
Defining the Solute: More Than Just Dissolving
A solute, in its simplest definition, is the component of a solution that is present in a smaller amount and gets dissolved by the solvent. This dissolution process results in a homogenous mixture where the solute particles are dispersed evenly throughout the solvent, creating a uniform composition. Understanding the solute's nature is crucial for comprehending the properties and behaviour of the resulting solution.
Key Characteristics of Solutes:
-
Solubility: This is perhaps the most critical property of a solute. Solubility refers to the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. High solubility indicates that a large amount of solute can dissolve readily, while low solubility implies limited dissolution. Factors affecting solubility include the nature of the solute and solvent (polarity, intermolecular forces), temperature, and pressure.
-
Polarity: The polarity of a solute significantly influences its solubility. Polar solutes, possessing a net dipole moment due to an uneven distribution of charge, tend to dissolve well in polar solvents like water. Conversely, nonpolar solutes, with evenly distributed charges, dissolve readily in nonpolar solvents like oil. This principle is often summarized as "like dissolves like."
-
Particle Size: The size of solute particles affects the rate of dissolution. Smaller particles have a larger surface area exposed to the solvent, leading to faster dissolution compared to larger particles. This is why grinding a solid solute into a fine powder often accelerates the dissolution process.
-
State of Matter: Solutes can exist in various states – solid, liquid, or gas. For instance, sugar (solid) dissolves in water (liquid), alcohol (liquid) dissolves in water (liquid), and carbon dioxide (gas) dissolves in water (liquid) to form carbonated beverages.
Types of Solutes and Their Applications:
The world of solutes is diverse, encompassing a vast range of substances with a multitude of applications. Here, we explore some key categories:
1. Ionic Solutes:
These solutes are composed of ions, electrically charged atoms or molecules. When dissolved in a polar solvent like water, ionic compounds dissociate into their constituent ions, resulting in a conductive solution. Examples include table salt (NaCl), which dissociates into Na⁺ and Cl⁻ ions, and other salts like potassium chloride (KCl) and calcium chloride (CaCl₂).
- Applications: Ionic solutes find extensive use in various industries. They are critical in electrolyte solutions in batteries, playing a crucial role in the electrochemical reactions that generate electricity. They also play essential roles in biological systems, regulating fluid balance and nerve impulse transmission. In food processing, ionic solutes such as salt are utilized for preserving food and enhancing taste.
2. Molecular Solutes:
These solutes are composed of molecules held together by covalent bonds. Unlike ionic solutes, they typically do not dissociate into ions upon dissolution. Examples include sugar (sucrose), ethanol, and glucose.
- Applications: Molecular solutes have diverse applications. Sugars like sucrose are vital components of food and beverages, providing sweetness and energy. Ethanol is a key component of alcoholic beverages and also serves as a solvent in many chemical processes. Glucose is a fundamental energy source for living organisms.
3. Gaseous Solutes:
Gases can also act as solutes, dissolving in liquid solvents or even other gases. The solubility of gases is significantly affected by temperature and pressure. Carbon dioxide, oxygen, and nitrogen are common gaseous solutes.
- Applications: Dissolved gases play crucial roles in various systems. Oxygen dissolved in water is essential for aquatic life. Carbon dioxide dissolved in water forms carbonic acid, contributing to the acidity of rainwater and oceans. Dissolved gases are also utilized in various industrial processes, such as carbonation of beverages and chemical synthesis.
4. Metallic Solutes:
Certain metals can dissolve in liquid metals, forming metallic solutions or alloys. These alloys often possess enhanced properties compared to their constituent metals. Examples include brass (copper and zinc) and bronze (copper and tin).
- Applications: Metallic solutes are crucial in materials science, contributing to the creation of alloys with improved strength, durability, and other desirable properties. They are used extensively in construction, manufacturing, and aerospace industries.
Factors Influencing Solute Behavior:
The behavior of a solute in a solution is influenced by various factors, including:
1. Temperature:
Temperature significantly impacts the solubility of many solutes. For most solid solutes, solubility increases with increasing temperature. However, the solubility of gases generally decreases with increasing temperature.
2. Pressure:
Pressure primarily affects the solubility of gaseous solutes. An increase in pressure increases the solubility of gases, according to Henry's Law. This principle explains why carbonated beverages fizz when opened, as the pressure is released, and the dissolved carbon dioxide escapes.
3. Intermolecular Forces:
The strength of intermolecular forces between solute and solvent molecules plays a critical role in determining solubility. Stronger interactions between solute and solvent lead to greater solubility. This is why polar solutes dissolve well in polar solvents due to strong dipole-dipole interactions or hydrogen bonding.
4. Solvent Properties:
The nature of the solvent itself greatly influences solute behavior. Polar solvents like water are good solvents for polar and ionic solutes, while nonpolar solvents are better for nonpolar solutes.
Solute Concentration: Expressing the Amount
The amount of solute present in a solution is referred to as its concentration. Several methods exist to express concentration, including:
- Molarity (M): Moles of solute per liter of solution.
- Molality (m): Moles of solute per kilogram of solvent.
- Normality (N): Equivalents of solute per liter of solution.
- Mass Percent (%): Mass of solute per 100 grams of solution.
- Parts per million (ppm) and parts per billion (ppb): Units often used for very dilute solutions.
Understanding solute concentration is vital for accurately preparing solutions and interpreting experimental results across various scientific disciplines.
The Importance of Solutes in Various Fields:
Solutes are fundamental components in numerous fields, playing critical roles in various processes:
1. Chemistry:
Solutes are integral to numerous chemical reactions and processes. They are used as reactants, catalysts, and solvents in various chemical syntheses. The understanding of solute behavior is crucial for optimizing chemical reactions and controlling product formation.
2. Biology:
Biological systems rely heavily on solutions and solutes. Ions such as sodium, potassium, and calcium are critical for nerve impulse transmission and muscle contraction. Glucose and other sugars are essential energy sources for living organisms. Proteins and other biomolecules dissolve in bodily fluids, carrying out vital functions.
3. Medicine:
Many pharmaceuticals are administered as solutions, allowing for controlled drug delivery. Intravenous fluids contain various solutes to maintain electrolyte balance and hydration. The solubility and bioavailability of drugs are crucial factors affecting their efficacy.
4. Environmental Science:
The concentration of various solutes in water bodies is a critical indicator of water quality. Excess nutrients like nitrates and phosphates can cause eutrophication, leading to harmful algal blooms. Heavy metal contamination poses significant risks to aquatic life and human health. Understanding solute behavior is crucial for environmental monitoring and remediation efforts.
Conclusion: The Unsung Heroes of Solutions
The solute, often overlooked, is a pivotal component in the vast world of solutions. Its properties, behavior, and interactions with the solvent dictate the characteristics and applications of the resulting solution. From the simplest sugar solution to complex biological systems, solutes play an indispensable role across various scientific fields and everyday life. A deeper understanding of solutes is crucial for advancements in chemistry, biology, medicine, environmental science, and many other disciplines, emphasizing their importance as the unsung heroes of solutions.
Latest Posts
Latest Posts
-
Electric Field Due To Ring Of Charge
Mar 31, 2025
-
Clusters Of Neuron Cell Bodies In The Pns Are Called
Mar 31, 2025
-
Solving Square Root And Other Radical Equations
Mar 31, 2025
-
What Are Characteristics Of A Virus
Mar 31, 2025
-
The Adrenal Glands Are Attached Superiorly To Which Organ
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Substance That Is Being Dissolved By A Solvent. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
