Chains Of Carbon Atoms Bonded To Hydrogen Atoms
Muz Play
Mar 31, 2025 · 7 min read
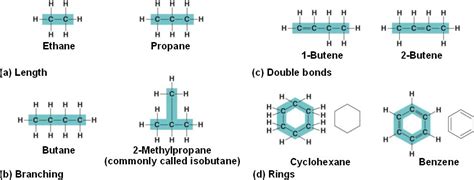
Table of Contents
Chains of Carbon Atoms Bonded to Hydrogen Atoms: Exploring the World of Hydrocarbons
Chains of carbon atoms bonded to hydrogen atoms—also known as hydrocarbons—form the fundamental building blocks of organic chemistry. Their seemingly simple structure belies an astonishing diversity of properties and applications, impacting everything from the energy sector to the pharmaceutical industry. Understanding these chains, their variations, and their chemical behavior is crucial for grasping the vast landscape of organic molecules. This article delves deep into the fascinating world of hydrocarbons, exploring their structure, classification, properties, and significance.
The Foundation: Carbon's Unique Bonding
Carbon's unique ability to form four strong covalent bonds is the cornerstone of organic chemistry. This tetravalency allows carbon atoms to bond with each other extensively, creating chains, branches, and rings of various lengths and complexities. The addition of hydrogen atoms completes the carbon's bonding capacity, resulting in the diverse array of hydrocarbon molecules. This simple combination of carbon and hydrogen, however, gives rise to an incredibly rich and complex chemistry.
Carbon-Carbon Bonds: The Backbone of Hydrocarbons
The strength and stability of carbon-carbon bonds are essential to the existence of long hydrocarbon chains. These bonds can be single, double, or triple bonds, significantly influencing the molecule's geometry, reactivity, and overall properties.
-
Single Bonds (Alkanes): Single carbon-carbon bonds allow for free rotation around the bond axis, leading to flexible and relatively unreactive chains. These saturated hydrocarbons, also known as alkanes, are the foundation upon which other hydrocarbon structures are built.
-
Double Bonds (Alkenes): Double bonds, composed of one sigma bond and one pi bond, restrict rotation around the bond axis, resulting in a rigid planar structure. This rigidity significantly impacts the molecule's shape and reactivity, making alkenes more reactive than alkanes.
-
Triple Bonds (Alkynes): Triple bonds, consisting of one sigma bond and two pi bonds, lead to a linear structure. Alkynes, possessing triple bonds, are even more reactive than alkenes due to the presence of two pi bonds.
Classifying Hydrocarbons: A Systematic Approach
Hydrocarbons are broadly classified into two main categories based on the presence or absence of cyclic structures:
1. Aliphatic Hydrocarbons: Open-Chain Structures
Aliphatic hydrocarbons are characterized by open-chain structures, lacking any ring formation. They are further subdivided into three groups:
-
Alkanes (Saturated Hydrocarbons): Alkanes contain only single carbon-carbon bonds. They are relatively unreactive and are often used as fuels and solvents. The simplest alkane is methane (CH₄), followed by ethane (C₂H₆), propane (C₃H₈), butane (C₄H₁₀), and so on. The general formula for alkanes is CₙH₂ₙ₊₂, where 'n' represents the number of carbon atoms.
-
Alkenes (Unsaturated Hydrocarbons): Alkenes possess at least one carbon-carbon double bond. The presence of the double bond introduces unsaturation and increased reactivity. They readily undergo addition reactions, making them crucial building blocks in polymer synthesis. Ethene (C₂H₄), also known as ethylene, is the simplest alkene.
-
Alkynes (Unsaturated Hydrocarbons): Alkynes contain at least one carbon-carbon triple bond. Their high reactivity is attributed to the presence of two pi bonds. Ethyne (C₂H₂), also known as acetylene, is the simplest alkyne, used extensively in welding due to its high heat of combustion.
2. Aromatic Hydrocarbons: Cyclic Structures with Delocalized Electrons
Aromatic hydrocarbons are characterized by the presence of a benzene ring or related structures. Benzene (C₆H₆) is the quintessential aromatic hydrocarbon, featuring a cyclic structure with delocalized pi electrons, resulting in exceptional stability. This delocalization leads to unique properties and reactivity distinct from aliphatic hydrocarbons.
-
Benzene and its Derivatives: Benzene's unique stability is due to the resonance stabilization of its delocalized electrons. Derivatives of benzene, where one or more hydrogen atoms are replaced with other groups, form a vast class of compounds with diverse applications in various industries.
-
Polycyclic Aromatic Hydrocarbons (PAHs): PAHs consist of multiple fused benzene rings. They are often found in coal tar, combustion products, and are known for their potential carcinogenic properties.
Properties of Hydrocarbons: A Spectrum of Characteristics
The properties of hydrocarbons vary significantly depending on their structure, chain length, and the type of carbon-carbon bonds present. Key properties include:
Physical Properties:
-
Boiling Point and Melting Point: Boiling and melting points generally increase with increasing chain length and molecular weight. Branched-chain alkanes have lower boiling points than their straight-chain isomers.
-
Solubility: Hydrocarbons are generally nonpolar and insoluble in water. They are soluble in nonpolar solvents like other hydrocarbons.
-
Density: Most hydrocarbons are less dense than water.
Chemical Properties:
-
Combustion: Hydrocarbons readily undergo combustion in the presence of oxygen, releasing large amounts of heat. This property makes them valuable fuels.
-
Reactivity: The reactivity of hydrocarbons depends largely on the type of carbon-carbon bonds present. Alkanes are relatively unreactive, while alkenes and alkynes are much more reactive, readily participating in addition reactions. Aromatic hydrocarbons show unique reactivity patterns dictated by their delocalized pi electron system.
Applications of Hydrocarbons: A Wide-Ranging Impact
Hydrocarbons are ubiquitous in our modern world, with applications spanning various industries:
Energy Sector:
-
Fuels: Alkanes, particularly methane, propane, butane, and octane, are essential components of natural gas and gasoline, serving as primary energy sources globally.
-
Petrochemicals: The cracking and refining of petroleum yields various hydrocarbons used as building blocks for plastics, synthetic fibers, and other materials.
Materials Science:
-
Plastics: Polyethylene, polypropylene, and polystyrene, all derived from alkenes, are extensively used in packaging, construction, and a vast array of everyday products.
-
Synthetic Fibers: Many synthetic fibers, like nylon and polyester, are made from hydrocarbons.
-
Solvents: Certain hydrocarbons are used as solvents in various industrial processes.
Pharmaceuticals and Other Industries:
-
Pharmaceuticals: Many pharmaceuticals are based on hydrocarbon skeletons, modified with functional groups to achieve desired biological activity.
-
Lubricants: Some hydrocarbons are used as lubricants in machinery due to their low viscosity and high resistance to degradation.
-
Cosmetics and Personal Care Products: Some hydrocarbons are used in cosmetics and personal care products as solvents and emulsifiers.
Isomerism in Hydrocarbons: Exploring Structural Variations
Isomerism is a crucial concept in understanding the diversity of hydrocarbons. Isomers are molecules with the same molecular formula but different structural arrangements. This leads to significant variations in their properties and reactivity.
Types of Isomerism:
-
Structural Isomerism: This involves differences in the connectivity of atoms within the molecule. For example, butane (C₄H₁₀) exists as two structural isomers: n-butane (a straight chain) and isobutane (a branched chain).
-
Stereoisomerism: This involves differences in the spatial arrangement of atoms within the molecule, without altering the connectivity. Geometric isomerism (cis-trans isomerism) and optical isomerism are examples of stereoisomerism found in alkenes and more complex hydrocarbons.
Environmental Concerns and Sustainable Practices
While hydrocarbons are vital for modern society, their extraction, processing, and combustion contribute significantly to environmental concerns:
-
Greenhouse Gas Emissions: The combustion of hydrocarbons releases carbon dioxide, a major greenhouse gas contributing to climate change.
-
Air Pollution: The incomplete combustion of hydrocarbons produces pollutants such as carbon monoxide, particulate matter, and nitrogen oxides, impacting air quality and human health.
-
Oil Spills: Oil spills from extraction and transportation activities cause severe environmental damage to marine ecosystems.
Sustainable practices, such as developing renewable energy sources, improving energy efficiency, and developing bio-based alternatives to petroleum-derived hydrocarbons, are essential to mitigate the environmental impact of hydrocarbon use.
Conclusion: A Dynamic Field of Study
The study of hydrocarbon chains is a dynamic and ever-evolving field. As our understanding of their structure, properties, and reactivity deepens, so too does our ability to develop new technologies and applications. From providing energy to shaping the materials that surround us, hydrocarbons remain fundamental to modern society. However, the crucial need for sustainable practices and responsible resource management remains paramount to ensuring a balanced approach to harnessing their immense potential while mitigating their environmental impacts. Further research into alternative fuel sources, efficient hydrocarbon utilization, and environmentally friendly disposal methods is essential for a more sustainable future. The continued exploration of hydrocarbon chemistry promises further discoveries and innovative applications, shaping the landscape of science and technology for years to come.
Latest Posts
Latest Posts
-
Two Different Ionic Compounds Each Contain
Apr 02, 2025
-
Part Ii Equilibria Involving Sparingly Soluble Salts
Apr 02, 2025
-
Adding Strong Acid To A Buffer
Apr 02, 2025
-
The Most Reactive Group In The Periodic Table
Apr 02, 2025
-
How To Write Quadratic Equation From Graph
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Chains Of Carbon Atoms Bonded To Hydrogen Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
